Abstract
Introduction
The progression of amyotrophic lateral sclerosis (ALS) leads to a decline of the nutritional status that represents an independent prognostic factor for survival. Recent studies recognize the muscle tissue as an endocrine organ able to release several molecules, called myokines. Among them, irisin seems to be involved in the regulation of metabolism, body weight and development and function of the nervous system.
Objectives
(1) To evaluate irisin serum levels in patients with ALS, with comparison to healthy subjects; (2) to assess the possible association of circulating irisin levels of ALS patients with the metabolic status, clinical and biochemical features.
Methods
We performed an observational, cross-sectional study in 50 ALS patients and 32 age- and sex-comparable healthy controls. Patients underwent to a complete set of neurological, pulmonary and nutritional evaluations. Serum irisin concentration was measured by enzyme immunoassay. According to indirect calorimetry, ALS patients were divided into a normo-metabolic patient group (n = 24) and a hyper-metabolic patient group (n = 26).
Results
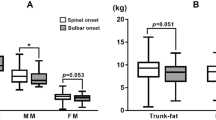
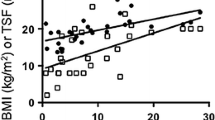
ALS patients showed significantly higher serum irisin levels compared to healthy subjects (51.0 ± 37.8 vs 13.1 ± 2.2 ng/mL, p < 0.0001). Hyper-metabolic ALS patients displayed higher serum irisin levels compared to normo-metabolic ALS patients and healthy controls (p < 0.0009 and p < 0.0001, respectively). Serum irisin levels showed significant association with the ALSFRS-R (β=-1.18, p = 0.042), Forced Vital Capacity (β = − 0.64, p = 0.013), Fat Mass (β=-1.44, p = 0.034), pCO2 arterial blood levels (β = 2.67, p = 0.003), HCO3− arterial blood levels (β = 5.44, p = 0.001) and Free Fat Mass (β = 1.07, p = 0.025) adjusted for sex, age and metabolic status.
Conclusions
ALS patients with impaired metabolic status showed higher serum irisin levels compared to normo-metabolic ALS patients and healthy subjects. Irisin levels were also negatively correlated with the extent of functional and respiratory impairment, due to as yet unknown causes, being more elevated in patients with greater disability.



Similar content being viewed by others
References
Kiernan MC, Vucic S, Cheah BC et al (2011) Amyotrophic lateral sclerosis. Lancet 377:942–955. https://doi.org/10.1016/S0140-6736(10)61156-7
Desport JC, Preux PM, Truong TC, Vallat JM, Sautereau D, Couratier P (1999) Nutritional status is a prognostic factor for survival in ALS patients. Neurology 53:1059–1063
Marin B, Desport JC, Kajeu P et al (2011) Alteration of nutritional status at diagnosis is a prognostic factor for survival of amyotrophic lateral sclerosis patients. J Neurol Neurosurg Psychiatry 82:628–634. https://doi.org/10.1136/jnnp.2010.211474
Bouteloup C, Desport JC, Clavelou P et al (2009) Hypermetabolism in ALS patients: an early and persistent phenomenon. J Neurol 256:1236–1242. https://doi.org/10.1007/s00415-009-5100-z
Desport JC, Preux PM, Magy L et al (2001) Factors correlated with hypermetabolism in patients with amyotrophic lateral sclerosis. Am J Clin Nutr 74:328–334
Desport JC, Torny F, Lacoste M, Preux PM, Couratier P (2005) Hypermetabolism in ALS: correlations with clinical and paraclinical parameters. Neurodegener Dis 2:202–207. https://doi.org/10.1159/000089626
Steyn FJ, Ioannides ZA, van Eijk RPA et al (2018) Hypermetabolism in ALS is associated with greater functional decline and shorter survival. J Neurol Neurosurg Psychiatry. https://doi.org/10.1136/jnnp-2017-317887
Jésus P, Fayemendy P, Nicol M et al (2018) Hypermetabolism is a deleterious prognostic factor in patients with amyotrophic lateral sclerosis. Eur J Neurol 25:97–104. https://doi.org/10.1111/ene.13468
Desport JC, Preux PM, Truong CT, Courat L, Vallat JM, Couratier P (2000) Nutritional assessment and survival in ALS patients. Amyotroph Lateral Scler Other Motor Neuron Disord 1:91–96
Febbraio MA, Pedersen BK (2005) Contraction-induced myokine production and release: is skeletal muscle an endocrine organ? Exerc Sport Sci Rev 33:114–119
Pedersen BK, Febbraio MA (2012) Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 8:457–465. https://doi.org/10.1038/nrendo.2012.49
Boström P, Wu J, Jedrychowski MP et al (2012) A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481:463–468. https://doi.org/10.1038/nature10777
Brenmoehl J, Albrecht E, Komolka K et al (2014) Irisin is elevated in skeletal muscle and serum of mice immediately after acute exercise. Int J Biol Sci 10:338–349. https://doi.org/10.7150/ijbs.7972
Huh JY, Siopi A, Mougios V, Park KH, Mantzoros CS (2015) Irisin in response to exercise in humans with and without metabolic syndrome. J Clin Endocrinol Metab 100:E453–E457. https://doi.org/10.1210/jc.2014-2416
Huh JY, Mougios V, Kabasakalis A et al (2014) Exercise-induced irisin secretion is independent of age or fitness level and increased irisin may directly modulate muscle metabolism through AMPK activation. J Clin Endocrinol Metab 99:E2154–E2161. https://doi.org/10.1210/jc.2014-1437
Norheim F, Langleite TM, Hjorth M et al (2014) The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J 281:739–749. https://doi.org/10.1111/febs.12619
Wrann CD, White JP, Salogiannnis J et al (2013) Exercise induces hippocampal BDNF through a PGC 1α/FNDC5 pathway. Cell Metab 18:649–659. https://doi.org/10.1016/j.cmet.2013.09.008
Zhang J, Zhang W (2016) Can irisin be a linker between physical activity and brainfunction? Biomol Concepts 7:253–258. https://doi.org/10.1515/bmc-2016-0012
Liu JJ, Wong MD, Toy WC et al. (20123) Lower circulating irisin is associated with type 2 diabetes mellitus. J Diabetes Complications 27:365–369. https://doi.org/10.1016/j.jdiacomp.2013.03.002
Pardo M, Crujeiras AB, Amil M et al (2014) Association of irisin with fat mass, resting energy expenditure, and daily activity in conditions of extreme body mass index. Int J Endocrinol 2014:857270. https://doi.org/10.1155/2014/857270
Andersen PM, Borasio GD, Dengler R et al (2005) EFNS task force on management of amyotrophic lateral sclerosis: guidelines for diagnosing and clinical care of patients and relatives. Eur J Neurol 12:921–938. https://doi.org/10.1111/j.1468-1331.2005.01351.x
Brooks BR, Miller RG, Swash M, Munsat TL (2000) World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1:293–299
Cedarbaum JM, Stambler N, Malta E et al (1999) The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci 169:13–21
Harris JA, Benedict FG (1918) A Biometric Study of Basal Metabolism in Man. Proc Natl Acad Sci USA 4:370–373
Maldonado G, Greenland S (1993) Simulation study of confounder-selection strategies. Am J Epidemiol 138:923–936
Copper A, Weekes TJ (1983) Data, models, and statistical analysis, Rowman & Littlefield. ISBN: 0-389-20383-1, pp 50–51
Mitsumoto H, Hanson MR, Chad DA (1988) Amyotrophic lateral sclerosis. Recent advances in pathogenesis and therapeutic trials. Arch Neurol 45:189–202
Zhang RZ, Gascon R, Miller RG et al (2005) Evidence for systemic immune system alterations in sporadic amyotrophic lateral sclerosis (sALS). J Neuroimmunol 159:215–224. https://doi.org/10.1016/j.jneuroim.2004.10.009
Appel SH (2006) Is ALS a systemic disorder? Evidence from muscle mitochondria. Exp Neurol 198:1–3. https://doi.org/10.1016/j.expneurol.2005.12.025
Wong M, Martin LJ (2010) Skeletal muscle-restricted expression of human SOD1 causes motor neuron degeneration in transgenic mice. Hum Mol Genet 19:2284–2302. https://doi.org/10.1093/hmg/ddq106
Ehinger JK, Morota S, Hansson MJ, Paul G, Elmér E (2015) Mitochondrial dysfunction in blood cells from amyotrophic lateral sclerosis patients. J Neurol 262:1493–1503. https://doi.org/10.1007/s00415-015-7737-0
Dupuis L, Oudart H, Rene F, Gonzalez de Aguilar JL, Loeffl er JP (2004) Evidence for defective energy homeostasis in amyotrophic lateral sclerosis: benefit of a high-energy diet in a transgenic mouse model. Proc Natl Acad Sci USA 101:11159–11164. https://doi.org/10.1073/pnas.0402026101
Funalot B, Desport JC, Sturtz F, Camu W, Couratier P (2009) High metabolic level in patients with familial amyotrophic lateral sclerosis. Amyotroph Lateral Scler 10:113–117. https://doi.org/10.1080/17482960802295192
Vaisman N, Lusaus M, Nefussy B et al (2009) Do patients with amyotrophic lateral sclerosis (ALS) have increased energy needs? J Neurol Sci 279:26–29. https://doi.org/10.1016/j.jns.2008.12.027
Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V (1996) Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic lateral sclerosis/Riluzole Study Group II. Lancet 347:1425–1431
Wijesekera LC, Leigh PN (2009) Amyotrophic lateral sclerosis. Orphanet J Rare Dis 4:3. https://doi.org/10.1186/1750-1172-4-3
Kasarskis EJ, Berryman S, Vanderleest JG, Schneider AR, McClain CJ (1996) Nutritional status of patients with amyotrophic lateral sclerosis: relation to the proximity of death. Am J Clin Nutr 63:130–137
Chiang PM, Ling J, Jeong YH, Price DL, Aja SM, Wong PC (2010) Deletion of TDP-43 down-regulates Tbc1d1, a gene linked to obesity, and alters body fat metabolism. Proc Natl Acad Sci USA 107:16320–16324. https://doi.org/10.1073/pnas.1002176107
Xu YF, Gendron TF, Zhang YJ et al (2010) Wild-type human TDP-43 expression causes TDP-43 phosphorylation, mitochondrial aggregation, motor deficits, and early mortality in transgenic mice. J Neurosci 30:10851–10859. https://doi.org/10.1523/JNEUROSCI.1630-10.2010
Crugnola V, Lamperti C, Lucchini V et al (2010) Mitochondrial respiratory chain dysfunction in muscle from patients with amyotrophic lateral sclerosis. Arch Neurol 67:849–854. https://doi.org/10.1001/archneurol.2010.128
Echaniz-Laguna A, Zoll J, Ribera F et al (2002) Mitochondrial respiratory chain function in skeletal muscle of ALS patients. Ann Neurol 52:623–627. https://doi.org/10.1002/ana.10357
Echaniz-Laguna A, Zoll J, Ponsot E et al (2006) Muscular mitochondrial function in amyotrophic lateral sclerosis is progressively altered as the disease develops: a temporal study in man. Exp Neurol 198:25–30. https://doi.org/10.1016/j.expneurol.2005.07.020
Lanfranconi F, Ferri A, Corna G et al (2017) Inefficient skeletal muscle oxidative function flanks impaired motor neuron recruitment in Amyotrophic Lateral Sclerosis during exercise. Sci Rep 7:2951. https://doi.org/10.1038/s41598-017-02811-z
Zhao W, Varghese M, Yemul S et al (2011) Peroxisome proliferator activator receptor gamma coactivator-1alpha (PGC-1a) improves motor performance and survival in a mouse model of amyotrophic lateral sclerosis. Mol Neurodegener 6:51. https://doi.org/10.1186/1750-1326-6-51
Da Cruz S, Parone PA, Lopes VS et al (2012) Elevated PGC-1 α activity sustains mitochondrial biogenesis and muscle function without extending survival in a mouse model of inherited ALS. Cell Metab 15:778–786. https://doi.org/10.1016/j.cmet.2012.03.019
Aronis KN, Moreno M, Polyzos SA et al (2015) Circulating irisin levels and coronary heart disease: association with future acute coronary syndrome and major adverse cardiovascular events. Int J Obes (Lond) 39:156–161. https://doi.org/10.1038/ijo.2014.101
Lopez-Legarrea P, De La Iglesia R, Crejeiras AB et al (2014) Higher baseline irisin concentrations are associated with greater reductions in glycemia and insulinemia af- ter weight loss in obese subjects. Nutr Diabetes 4:e110. https://doi.org/10.1038/nutd.2014.7
Gannon NP, Vaughan RA, Garcia-Smith R, Bisoffi M, Trujillo KA (2015) Effects of the exercise-inducible myokine irisin on malignant and non-malignant breast epithelial cell behavior in vitro. Int J Cancer 136:E197–E202. https://doi.org/10.1002/ijc.29142
Ebert T, Focke D, Petroff D et al (2014) Serum levels of the myokine irisin in relation to metabolic and renal function. Eur J Endocrinol 170:501–506. https://doi.org/10.1530/EJE-13-1053
Phillips C, Baktir MA, Srivatsan M, Salehi A (2014) Neuroprotective effects of physical activity on the brain: a closer look at trophic factor signaling. Front Cell Neurosci 8:170. https://doi.org/10.3389/fncel.2014.00170
Tremolizzo L, Pellegrini A, Conti E et al (2016) BDNF serum levels with respect to multidimensional assessment in amyotrophic lateral sclerosis. Neurodegener Dis 16:192–198. https://doi.org/10.1159/000441916
Acknowledgements
We thank our patients and their caregivers for their support of our study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no financial support for the conduct of the study. Dr Lunetta served on a scientific advisory board for Neuraltus and Italfarmaco and has received funds from Agenzia Italiana per la Ricerca sulla SLA (ARISLA) and Ministry of Health (CCM2011). The other authors report no conflicts of interest.
Ethical standards
The study has been approved by our Ethics Committee. All data were gathered after informed consent was obtained from each participant, in accordance with specific national laws and the ethics standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Rights and permissions
About this article
Cite this article
Lunetta, C., Lizio, A., Tremolizzo, L. et al. Serum irisin is upregulated in patients affected by amyotrophic lateral sclerosis and correlates with functional and metabolic status. J Neurol 265, 3001–3008 (2018). https://doi.org/10.1007/s00415-018-9093-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-9093-3