Abstract
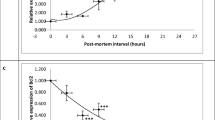
Recent biochemical, metabolic, and molecular profiles of various body fluids showed more accurate correlation to the postmortem interval than the traditional physical examination. Our study aimed to evaluate time passed since death in relation to oxidative stress markers, HMGB1 genetic expression, histopathological examination, and BCL2 immunohistochemical analysis in major organs (heart, kidney, and testis). Forty-two adult male rats were included and randomly divided into seven equal groups. After sacrification, the rodents were kept at room temperature and major organs were obtained at 0, 12, 24, 48, 72, 96, and 120 h. Malonaldehyde (MDA), superoxide dismutase (SOD), reduced glutathione (GSH) tissue levels, High mobility group box 1 protein (HMGB1) gene expression, histopathological, and B cell lymphoma 2 (BCL2) immunohistochemical expressions were analyzed. Postmortem interval was correlated to different tissue levels of MDA, SOD, and GSH. HMGB1 showed enhanced postmortem gene expression with a peak at 48 h after death. Obvious time-dependent histopathological changes were observed in all the examined organs. Dilated spaces, extravasation, and fragmentation scores in heart specimens were higher at 96 and 120 h compared with the other groups. Renal changes in the form of shrunken glomeruli, loss of tubular epithelium, and hyalinization and testicular findings in the form of epithelial detachment, vacuolation, and loss of sperms started at 72 h postmortem. BCL2 expression began to decrease 24 h and became negative at 96 h after death. In conclusion, HMGB1 gene expression can be used for estimation of time passed since death as it shows time-dependent changes in the form of a progressive increase with a peak at 48 h then it begins to decline. Oxidants and antioxidants are correlated to PMI until 120 h after death. Histopathological changes in the heart, kidney, and testis are also time-dependent until the 5th day after death. BCL2 immunohistochemical expression begins to decline 24 h until 96 h after death when it becomes negative.









Similar content being viewed by others
References
Chandrakanth HV, Kanchan T, Balaraj BM, Virupaksha HS, Chandrashekar TN (2013) Postmortem vitreous chemistry— an evaluation of sodium; potassium and chloride levels in estimation of time since death (during the first 36 h after death). J Forensic Legal Med 20(4):211–216
Madea B, Musshoff F (2007) Postmortem biochemistry. Forensic Sci Int 165:165–171
Poloz YO, O’Day DH (2009) Determining time of death: temperature-dependent postmortem changes in calcineurin a; MARCKS; CaMKII; and protein phosphatase 2A in mouse. Int J Legal Med 123(4):305–314
Tumram NK, Bardale RV, Dongre AP (2011) Postmortem analysis of synovial fluid and vitreous humour for determination of death interval: a comparative study. Forensic Sci Int 204:186–190
Clarkson PM, Thompson HS (2000) Antioxidants: what role do they play in physical activity and health. Am J Clin Nutr 72:637–646
Munoz Barus JI, Febrero-Bande M, Cadarso-Suarez C (2008) Flexible regression models for estimating postmortem interval (PMI) in forensic medicine. Stat Med 27:5026–5038
Javan GT, Can I, Finley SJ, Soni S (2015) The apoptotic thanatotranscriptome associated with the liver of cadavers. Forensic Sci Med Pathol 11:509–516
Magna M, Pisetsky DS (2014) The role of HMGB1 in the pathogenesis of inflammatory and autoimmune diseases. Mol Med (Cambridge, Mass) 20(1):138–146. https://doi.org/10.2119/molmed.2013.00164
Scaffidi P, Misteli T, Bianchi ME (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418:191–195
VanGuilder HD, Vrana KE, Freeman WM (2008) Twenty-five years of quantitative PCR for gene expression analysis. BioTechniques 44:619–626
Aziz NM, Elbassuoni EA, Kamel MY, Ahmed SM (2020) Hydrogen sulfide renal protective effects: possible link between hydrogen sulfide and endogenous carbon monoxide in a rat model of renal injury. Cell Stress Chaperones 25:211–221. https://doi.org/10.1007/s12192-019-01055-2
Ye Z, Lu W, Liang L, Tang M, Wang Y, Li Z, Zeng H, Wang A, Lin M, Huang L, Wang H, Hu H (2019) Mesenchymal stem cells overexpressing hepatocyte nuclear factor-4 alpha alleviate liver injury by modulating anti-inflammatory functions in mice. Stem Cell Res Ther 10(1):149. https://doi.org/10.1186/s13287-019-1260-7
Zhu BL, Ishikawa T, Michiue T, Li DR, Zhao D, Quan L, Oritani S, Bessho Y, Maeda H (2007) Postmortem serum catecholamine levels in relation to the cause of death. Forensic Sci Int 173:122–129
Pozhitkov AE, Neme R, Domazet-Losˇo T, Leroux BG, Soni S, Tautz D, Noble PA (2017) Tracing the dynamics of gene transcripts after organismal death. Open Biol 7:160267. https://doi.org/10.1098/rsob.160267
Abo El-Noor MM, Elhosary NM, Khedr NF, El-Desouky KI (2016) Estimation of early postmortem interval through biochemical and pathological changes in rat heart and kidney. Am J Forensic Med Pathol 37:40–46
Ozturk C, Sener MT, Sener E et al (2013) The investigation of damage in the muscle tissue with the oxidant/antioxidant balance and the extent of postmortem DNA damage in rats. Life Sci J 10(3):1631–1637
Kikuchi K, Kawahara KI, Biswas KK, Ito T, Tancharoen S, Shiomi N, Koda Y, Matsuda F, Morimoto Y, Oyama Y, Takenouchi K, Miura N, Arimura N, Nawa Y, Arimura S, Jie MX, Shrestha B, Iwata M, Mera K, Sameshima H, Ohno Y, Maenosono R, Tajima Y, Uchikado H, Kuramoto T, Nakayama K, Shigemori M, Yoshida Y, Hashiguchi T, Maruyama I (2010) HMGB1: a new marker for estimation of the postmortem interval. Exp Ther Med 1(1):109–111. https://doi.org/10.3892/etm_00000019 Epub 2010 Jan 1. PMID: 23136602; PMCID: PMC3490379
Mohamed AAR, Elbohi KM, Sharkawy NIE, Hassan MA (2017) Biochemical and apoptotic biomarkers as indicators of time elapsed since death in experimentally induced traumatic brain injury. SM J Forensic Res Criminol 1(2):1010
Tolbert M, Finley SJ, Visonà SD, Soni S, Osculati A, Javan GT (2018) The thanatotranscriptome: gene expression of male reproductive organs after death. Gene 675:191–196. https://doi.org/10.1016/j.gene.2018.06.090
Zdravković M, Kostov M, Stojanović M (2006) Identification of postmortem autolytic changes on the kidney tissue using PAS stained method. Med Biol 13(3):181–184
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. All procedures performed in the study involving animals were in accordance with the ethical standards of the institution at which the study was conducted.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Welson, N.N., Gaber, S.S., Batiha, G.ES. et al. Evaluation of time passed since death by examination of oxidative stress markers, histopathological, and molecular changes of major organs in male albino rats. Int J Legal Med 135, 269–280 (2021). https://doi.org/10.1007/s00414-020-02463-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-020-02463-1