Abstract
Parvovirus B19 (PVB19) commonly infects children and is usually asymptomatic. Lethal outcomes of PVB19 infection are unusual; nevertheless, the two cases reported here are rare examples of PVB19-induced hemophagocytic syndrome and myocarditis in infants and children. The two cases show the indisputable usefulness of immunohistochemistry and in situ hybridization in the detection of PVB19. In the death investigations, histopathological examinations provided stronger evidence than did serology or molecular biology. The cases also highlight the importance of forensic autopsy in vaccine-related death. As vaccine-related deaths are what people fear and may cause declines in vaccination rates, it is important to clarify deaths temporally or causally associated with vaccine administration.
Similar content being viewed by others
Introduction
Parvovirus B19 (PVB19) is a strict human pathogen in the family Parvoviridae [1]. PVB19 is transmitted by the respiratory route, blood transfusion, and transplacental infection from an acutely infected mother [2,3,4]. Children and adolescents younger than 15 years are the most affected age groups, and almost half of them have anti-parvovirus antibodies [5]. Patients with PVB19 infection show a wide range of clinical severity, ranging from asymptomatic to lethal. PVB19 commonly causes fifth disease, also known as erythema infectiosum, and the infection is frequently self-limiting. PVB19 can also cause arthropathy, transient aplastic crisis, and chronic red cell aplasia [1]. PVB19-associated hemophagocytic syndrome and myocarditis have been rarely reported in infants or children, and these findings were proven in previous publications by only serology or molecular biology without direct evidence [6,7,8,9].
Infectious disease is effectively prevented by immunization. Nevertheless, adverse events or even death following immunization are what people fear and may cause declines in vaccination rates [10]. Therefore, it is important to clarify the causal relationship between vaccine administration and death. In the present paper, we report two controversial cases of death occurring in close temporal proximity to vaccine administration. Due to the timing of vaccination, both cases underwent forensic autopsy. After investigation, we found that the deaths were temporally but not causally related to vaccine administration. Using multiplex real-time polymerase chain reaction (MRT-PCR), immunohistochemistry, and in situ hybridization, we confirmed PVB19-induced hemophagocytic syndrome and myocarditis, respectively.
Case reports
Case 1
A 7-year-old boy was hospitalized in November 2009 because of rash and fever after receiving a shot of AdimFlu-S (A/H1N1) vaccine. About 1 month later, he died. Figure 1 illustrates the whole course of disease and therapy. The boy had traveled to Malaysia and had contact with chameleons, peacocks, mice, and turtles 1 month prior. In the late course of disease, total blood count showed RBC 2.72 × 106/μL, WBC 32,920/μL, and platelets 52,000/μL. The coagulation screen was abnormal, with prothrombin time (PT) 15 s and partial thromboplastin time (PTT) 68.1 s. The liver function tests were abnormal, with total serum bilirubin 2.25 mg/dl, glutamate oxaloacetate transaminase (GOT) 736 U/L, and glutamate pyruvate transaminase (GPT) 137 U/L. There was diffuse intravascular coagulation (DIC), with D-Dimer > 35 μg/ml, fibrinogen > 0.035 mg/dl, and fibrin degradation products (FDP) 468.4 μg/mL. Serum showed high levels of triglycerides, lactic dehydrogenase, and ferritin at 178 mg/dl, 9560 U/I, and 142,874 ng/ml, respectively.
A complete autopsy was performed. Gross examination showed brainstem hemorrhage and cerebral edema, with an 8-cm hematoma and intraventricular hemorrhage. The boy suffered from bleeding tendency, including multiple petechiae and ecchymosis of the skin, sclera, conjunctivae, subgaleal soft tissue, lung surface, intestinal wall, and renal pelvis. Pulmonary edema, pleural effusion, hepatomegaly with steatosis, renal swelling, numerous enlarged mesenteric lymph nodes, and bloody ascites were observed.
Microscopically, the bone marrow showed distinct hemophagocytosis. Numerous macrophages with abundant foamy cytoplasm were evenly distributed in the bone marrow, engulfing blood cells and their precursor cells (Fig. 2a). The heart showed scattered tiny foci of myocardial damage. The lung showed intra-alveolar hemorrhage and edema with many foamy macrophages and occasional hyaline membranes. The liver showed significant microvesicular steatosis with small patches of necrosis and increased activities of Kupffer cells but no significant inflammation. The spleen showed lymphocytic depletion and congestion of sinuses with macrophage infiltration. The kidney showed acute tubular necrosis. The mesenteric lymph nodes showed necrotizing lymphadenitis with necrosis at the germinal centers adjacent to the cortex and nuclear degraded residues. The tonsils showed mild inflammation with slight infiltration of eosinophils and lymphocytes. Several foci of hemorrhage of internal organs were noted. The subcutaneous tissue of the palm and thenar surfaces exhibited fibrinoid microthrombi and hemorrhage consistent with disseminated intravascular coagulation. The subcutaneous tissues showed small atypical foamy macrophages. The hemorrhage areas near the brainstem presented numerous foamy macrophages without remarkable inflammation.
Case 2
An 11-month-old female infant with respiratory symptoms for 1 week was admitted to the pediatric clinic in October 2012. She was diagnosed with an upper respiratory tract infection and received a shot of MF59-adjuvanted influenza vaccine 9 days later. The following day, she was admitted to the hospital because of vomiting and dyspnea and had an episode of seizure on the way to the hospital. She was in cardiac arrest upon arrival to the emergency department and died despite vigorous resuscitation. Blood samples obtained right after the start of cardiopulmonary resuscitation showed a leukocyte count of 12,200 cells/mm3, with a lymphocyte proportion of 60% and a neutrophil proportion of 35%. Her platelet count was 336,000/mm3. The hemoglobin level was 8.13 mg/dl with a mean corpuscular volume of 68.8 femtoliters. The level of C-reactive protein was 0.09 mg/dl, and the alanine transaminase and aspartate transaminase levels were 12 and 36 IU/ml, respectively. The creatine kinase and creatine kinase MB levels were 69 and 24 IU/L, respectively. The troponin I level was 0.13 ng/mL.
A complete autopsy was performed. Gross examination showed an enlarged and pale heart with ventricular dilatation. The myocardium was edematous and pale, indicating suspected myocarditis. Swollen tonsils, hepatomegaly, and pulmonary edema with some petechiae on the lung surface were noticed. There was pleural effusion of 100 ml. There was no cellulitis over the vaccine injection site, and there was no vesicle, rash, or erythema over the whole skin, anus, and oral mucosa.
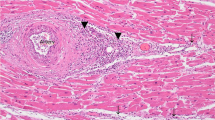
Microscopically, the heart demonstrated lymphocytic myocarditis. Myocardial fibers with conspicuous lymphocyte and plasma cell infiltration were found in cardiac tissue (Fig. 3a), consistent with the pathology of subacute myocarditis with early-stage fibrosis. The lung showed interstitial pneumonitis, and tissue presented with significant lymphocytic infiltration, especially around pulmonary vessels and bronchioles. There was no significant eosinophilic infiltration over the trachea. The hippocampus and medulla showed perivascular cuffing. The liver showed lymphocyte accumulation in the portal area, and there was microvascular fatty metamorphosis in the hepatocytes. The renal interstitium showed lymphocyte accumulation and germinal center formation. The white pulp and red pulp of spleen distributed evenly, and the red pulp was slightly congested. The adrenal medulla was congested with partial hemorrhage. There was tonsillitis with inflammatory cell infiltration in the epithelium. The mucosa of the colon showed lymphocyte accumulation and germinal center formation. The bone marrow demonstrated hemophagocytosis with many infected cells showing intranuclear inclusion bodies (Fig. 3b).
Case 2—a Myocarditis (× 100). Hematoxylin and eosin stain. b Many infected cells showed intranuclear inclusion bodies in the bone marrow (× 1000). Hematoxylin and eosin stain. c PVB19 was detected in macrophages by immunohistochemistry of myocardial tissue (× 400). d PVB19 was detected in bone marrow by in situ hybridization (× 200)
Methods
Autopsy procedures
A complete autopsy was performed by forensic pathologists. Tissue specimens from all major organs were fixed immediately in 10% neutral buffered formalin. Fixed tissues were hydrated in ethanol, cleared in xylene, and embedded in paraffin blocks. Tissue sections of 4 μm thick were prepared from formalin-fixed, paraffin-embedded (FFPE) blocks and used for hematoxylin-eosin (HE) staining and immunostaining.
For potential infectious etiology investigations, specimens of microbiological analysis were collected at autopsy by aseptic technique, including swabs, fresh tissue, blood culture, and serum. The microbiology analysis, including bacterial isolation, virus isolation and multiplex reverse transcription polymerase chain reaction, was performed by the Taiwan Centers for Disease Control (Table 1).
Immunohistochemistry
Four-micrometer sections were cut and mounted on silane-coated slides. The slides were heated to 65 °C for 20 min and then sequentially immersed in xylene (8 min, twice), absolute ethanol (50 s, twice), 95% ethanol (20 s, twice), and distilled water. Antigen retrieval was achieved with 0.1 mg/ml proteinase K (ENZ-33801, Enzo Life Science, USA) for 30 min at 37 °C. Then, the slides were covered with Ultra V protein block (TA-060-UB, Thermo Scientific, USA) for 10 min. The sections were incubated with anti-parvovirus antibody [R92F6] (1:20 dilution; ab50804, Abcam, England) for 1 h and visualized with Ultravision Quanto AP Detection System (TL-060-AL, Thermo Scientific, USA) and Permanent Fast Red Quanto Substrate System (TA-060-QAL, Thermo Scientific, USA). Subsequently, the slides were counterstained with hematoxylin and mounted with aqueous mounting medium.
In situ hybridization
Four-micrometer sections from paraffin-embedded blocks were deparaffinized in xylene, dehydrated, and predigested with 0.1 mg/ml proteinase K (ENZ-33801, Enzo Life Science, USA) for 30 min at 37 °C. The slides were washed with distilled water, rinsed in an ethanol series (70, 95, and 100%), and air-dried. The oligonucleotide probe sequence complementary to the gene encoding the NS protein was described previously [11]; 5′ digoxigenin-GATTCTCCTGAACTGGTCCC-3′ was synthesized (Purigo Biotech, Inc., Taipei, Taiwan). A glass coverslip was placed over the probe (10 ng/ml), and the slides were put into ThermoBrite (Abbott, USA), denatured for 2 min at 95 °C, and then hybridized for 16 h at 37 °C. On the following day, the coverslips were removed, and the slides were washed in 2X SSC solution containing detergent (0.1% NP-40, Sigma, USA) for 2 min at 73 °C and washed in the solution for 1 min at room temperature. Hybridization products were detected using an in situ hybridization detection system core kit for digoxigenin-labeled probe/streptavidin-alkaline phosphatase (DD131-60 K, BioGenex, CA). Paraffin-embedded sections of non-infected normal bone marrow were used as negative controls. Sections without probe were also included as controls for background staining.
Results and discussions
To determine a potential infectious etiology, screening was performed by MRT-PCR. The pathogens analyzed included herpesvirus type 1 to 8, influenza virus A and B, parainfluenza virus type 1 to 4, coronavirus, polyomavirus, chikungunya, dengue, West Nile virus, JE, adenovirus, rhinovirus, human metapneumovirus, respiratory syncytial virus, enterovirus, human parechovirus, human PVB19, bocavirus, Hendra virus, Nipah virus, mycoplasma, borrelia, Balamuthia, Acanthamoeba, Naegleria, and Toxoplasma (Table 1). In case 1, PVB19 was detected in bone marrow, heart, liver, spleen, lung, kidney, and basal ganglia (Table 2). In case 2, PVB19 was detected in trachea and throat swabs, as well as in fresh tissue of heart, lung, spleen, and liver and in blood specimens at a high viral load (Table 3). The bacterial isolation and virus isolation tests yielded no significant results. PVB19 was also detected in the myocardial tissue by immunohistochemistry in case 2. The positive signals were demonstrated in macrophages (Fig. 3c). Moreover, PVB19 was also detected in bone marrow by in situ hybridization in both cases (Figs. 2b and 3d).
PVB19-induced myocarditis has been reported since 1990 [12, 13]. Here, we report a case of an 11-month-old female infant with PVB19-induced fulminant lymphocytic myocarditis (case 2). It has been reported that PVB19 causes fatal myocarditis in immunocompetent adults [14,15,16], liver transplant recipients [17], and Brugada syndrome patients [18], but the most affected age groups of PVB19-associated fatal myocarditis are children (aged 3 to 11 years) [8, 9, 19,20,21,22,23], fetuses [24, 25], and infants [7]. Notably, the aforementioned reports demonstrated the relationship between myocarditis and PVB19 by indirect molecular and serology methods rather than by direct immunohistochemistry or in situ hybridization. This is precarious, because a recent study showed that the detection of PVB19 DNA was not sufficient for diagnosing myocarditis [26, 27]. Therefore, we used immunohistochemistry and in situ hybridization, in addition to microbiological examination, to confirm the pathogenesis of PVB19. The positive signals were demonstrated in macrophages by immunohistochemistry from myocardial tissue (Fig. 3c) and in red blood cell precursors by in situ hybridization from bone marrow (Fig. 3d).
We reported a PVB19 infection with DIC and hemophagocytic syndrome in a 7-year-old boy (case 1). PVB19 is one of the pathogens of virus-associated hemophagocytic syndrome [28], and it has been reported in pregnant individuals [29], transplant recipients [30], patients with acute lymphoblastic leukemia [31] or autoimmune disease [32], and immunocompetent adults [16, 33]. Nevertheless, PVB19-related hemophagocytic syndrome with DIC has been rarely reported [6, 34]. Anti-PVB19 immunoglobulin G (IgG), which produces long-term immunity in most patients, was detected in patient 1 on the 20th day after vaccine administration, but this patient died on the 31st day after vaccine administration. Such rapid clinical deterioration indicated an inhibited immune system, resulting in uncontrolled viral replication. During the hospitalization, this patient received prednisolone and intravenous immunoglobulin (IVIG). Etoposide-containing regimens combined with IVIG and prednisolone have been tried in pediatric hemophagocytic syndrome [35]. Although the initial use of IVIG may be beneficial in the treatment of PVB19-induced hemophagocytic syndrome [35], some people have a negative opinion [36, 37]. Therefore, there remains no definite opinion regarding prednisolone and IVIG in the treatment of hemophagocytic syndrome.
When these two patients were immunized, patient 1 was healthy, but patient 2 had rhinorrhea, an injected throat, nasal mucosal swelling, and a body temperature of 37.4 °C. Whether children with illnesses can be safely immunized depends on the severity of the illness [38, 39]. A mild illness is not a reason to delay or reschedule routine vaccinations [38], but a moderate to severe or acute illness is a precaution for the use of influenza vaccines [39]. As routine annual influenza vaccination is recommended for people aged 6 months or older without contraindications [39], administering the vaccination to patient 2 may be valuable. However, deferring the influenza vaccination did not result in missed opportunities for vaccination for patient 2, because vaccination even in December or later may be beneficial throughout the influenza season [39]. Therefore, the timing of vaccination could have been more careful in patient 2.
To the best of our knowledge, these are the first case reports using real-time PCR, immunohistochemistry, and in situ hybridization to confirm PVB19-induced hemophagocytic syndrome and myocarditis. PVB19 infection induced DIC and hemophagocytic syndrome in case 1 and caused fulminant lymphocytic myocarditis in case 2. Collectively, our data demonstrated different underlying pathophysiological mechanisms of the death from PVB19 infection, and the findings may serve as a reference for other studies of fatal PVB19 infection. Furthermore, vaccine administration was supposed to be related in both cases based on first impressions of temporal proximity. However, the temporal relationships between vaccination and lethal outcomes were essentially coincidental and prompted forensic autopsies. Our reports remind clinicians to avoid tunnel vision and highlight the importance of forensic autopsy in vaccine-related death.
References
Heegaard ED, Brown KE (2002) Human parvovirus B19. Clin Microbiol Rev 15:485–505
Chorba T, Coccia P, Holman RC, Tattersall P, Anderson LJ, Sudman J, Young NS, Kurczynski E, Saarinen UM, Moir R, Lawrence DN, Jason JM, Evatt B (1986) The role of parvovirus B19 in aplastic crisis and erythema infectiosum (fifth disease). J Infect Dis 154:383–393
Bell LM, Naides SJ, Stoffman P, Hodinka RL, Plotkin SA (1989) Human parvovirus B19 infection among hospital staff members after contact with infected patients. N Engl J Med 321:485–491. https://doi.org/10.1056/NEJM198908243210801
Dijkmans AC, de Jong EP, Dijkmans BA et al (2012) Parvovirus B19 in pregnancy: prenatal diagnosis and management of fetal complications. Curr Opin Obstet Gynecol 24:95–101. https://doi.org/10.1097/GCO.0b013e3283505a9d
Centers for Disease Control (CDC) (1989) Risks associated with human parvovirus B19 infection. MMWR Morb Mortal Wkly Rep 38:81–88 93–7
Kaya Z, Ozturk G, Gursel T, Bozdayi G (2005) Spontaneous resolution of hemophagocytic syndrome and disseminated intravascular coagulation associated with parvovirus b19 infection in a previously healthy child. Jpn J Infect Dis 58:149–151
Papadogiannakis N, Tolfvenstam T, Fischler B, Norbeck O, Broliden K (2002) Active, fulminant, lethal myocarditis associated with parvovirus B19 infection in an infant. Clin Infect Dis 35:1027–1031. https://doi.org/10.1086/342574
Dina J, Vabret A, Rambaud C, Checoury A, Gouarin S, Petitjean J, Freymuth F (2008) Fulminant myocarditis associated with parvovirus B19 infection in a child. J Clin Virol 42:70–71. https://doi.org/10.1016/j.jcv.2007.12.018
Koehl B, Oualha M, Lesage F, Rambaud C, Canioni D, Hubert P, Leruez-Ville M (2012) Fatal parvovirus B19 myocarditis in children and possible dysimmune mechanism. Pediatr Infect Dis J 31:418–421. https://doi.org/10.1097/INF.0b013e3182425786
Ropeik D (2013) How society should respond to the risk of vaccine rejection. Hum Vaccin Immunother 9:1815–1818. https://doi.org/10.4161/hv.25250
Yeh SP, Chiu CF, Lee CC, Peng CT, Kuan CY, Chow KC (2004) Evidence of parvovirus B19 infection in patients of pre-eclampsia and eclampsia with dyserythropoietic anaemia. Br J Haematol 126:428–433. https://doi.org/10.1111/j.1365-2141.2004.05043.x
Saint-Martin J, Choulot JJ, Bonnaud E, Morinet F (1990) Myocarditis caused by parvovirus. J Pediatr 116:1007–1008
Saint-Martin J, Bonnaud E, Morinet F, Choulot JJ, Mensire A (1991) Acute parvovirus myocarditis with fatal outcome. Pediatrie 46:597–599
Orth T, Herr W, Spahn T et al (1997) Human parvovirus B19 infection associated with severe acute perimyocarditis in a 34-year-old man. Eur Heart J 18:524–525
Bultmann BD, Klingel K, Sotlar K et al (2003) Fatal parvovirus B19-associated myocarditis clinically mimicking ischemic heart disease: an endothelial cell-mediated disease. Hum Pathol 34:92–95. https://doi.org/10.1053/hupa.2003.48
Bal A, Mishra B, Singh N, Das A, Jindal SK (2009) Fulminant parvovirus B19-associated pancarditis with haemophagocytic lympho-histiocytosis in an immunocompetent adult. APMIS 117:773–777. https://doi.org/10.1111/j.1600-0463.2009.02528.x
Jonetzko P, Graziadei I, Nachbaur K, Vogel W, Pankuweit S, Zwick R, Pachinger O, Poelzl G (2005) Fatal course of parvovirus B19-associated myocarditis in a female liver transplant recipient. Liver Transpl 11:463–466. https://doi.org/10.1002/lt.20375
Juhasz Z, Tiszlavicz L, Kele B, Terhes G, Deak J, Rudas L, Kereszty E (2014) Sudden cardiac death from parvovirus B19 myocarditis in a young man with Brugada syndrome. J Forensic Legal Med 25:8–13. https://doi.org/10.1016/j.jflm.2014.04.018
Enders G, Dotsch J, Bauer J, Nutzenadel W, Hengel H, Haffner D, Schalasta G, Searle K, Brown KE (1998) Life-threatening parvovirus B19-associated myocarditis and cardiac transplantation as possible therapy: two case reports. Clin Infect Dis 26:355–358
Beghetti M, Gervaix A, Haenggeli CA, Berner M, Rimensberger PC (2000) Myocarditis associated with parvovirus B19 infection in two siblings with merosin-deficient congenital muscular dystrophy. Eur J Pediatr 159:135–136
Murry CE, Jerome KR, Reichenbach DD (2001) Fatal parvovirus myocarditis in a 5-year-old girl. Hum Pathol 32:342–345. https://doi.org/10.1053/hupa.2001.22743
Rohayem J, Dinger J, Fischer R, Klingel K, Kandolf R, Rethwilm A (2001) Fatal myocarditis associated with acute parvovirus B19 and human herpesvirus 6 coinfection. J Clin Microbiol 39:4585–4587. https://doi.org/10.1128/JCM.39.12.4585-4587.2001
Dettmeyer R, Kandolf R, Baasner A, Banaschak S, Eis-Hubinger AM, Madea B (2003) Fatal parvovirus B19 myocarditis in an 8-year-old boy. J Forensic Sci 48:183–186
Respondek M, Bratosiewicz J, Pertynski T, Liberski PP (1997) Parvovirus particles in a fetal-heart with myocarditis: ultrastructural and immunohistochemical study. Arch Immunol Ther Exp 45:465–470
Hichijo A, Morine M (2014) A case of fetal parvovirus b19 myocarditis that caused terminal heart failure. Case Rep Obstet Gynecol 2014:463571. https://doi.org/10.1155/2014/463571
Schenk T, Enders M, Pollak S, Hahn R, Huzly D (2009) High prevalence of human parvovirus B19 DNA in myocardial autopsy samples from subjects without myocarditis or dilative cardiomyopathy. J Clin Microbiol 47:106–110. https://doi.org/10.1128/JCM.01672-08
Koepsell SA, Anderson DR, Radio SJ (2012) Parvovirus B19 is a bystander in adult myocarditis. Cardiovasc Pathol 21:476–481. https://doi.org/10.1016/j.carpath.2012.02.002
Wang YC, Liu DJ, Ma LN, Liu MJ, Sheng GY, Zhao XM (2015) Clinical features of childhood hemophagocytic syndrome and its association with human parvovirus B19 infection. Zhongguo Dang Dai Er Ke Za Zhi 17:26–30. https://doi.org/10.7499/j.issn.1008-8830.2015.01.006 [pii]
Mayama M, Yoshihara M, Kokabu T, Oguchi H (2014) Hemophagocytic lymphohistiocytosis associated with a parvovirus B19 infection during pregnancy. Obstet Gynecol 124:438–441. https://doi.org/10.1097/AOG.0000000000000385
Tavera M, Petroni J, Leon L, Minue E, Casadei D (2012) Reactive haemophagocytic syndrome associated with parvovirus B19 in a kidney-pancreas transplant patient. Nefrologia 32:125–126. https://doi.org/10.3265/Nefrologia.pre2011.Oct.11179
Matsubara K, Uchida Y, Wada T, Iwata A, Yura K, Kamimura K, Nigami H, Fukaya T (2011) Parvovirus B19-associated hemophagocytic lymphohistiocytosis in a child with precursor B-cell acute lymphoblastic leukemia under maintenance chemotherapy. J Pediatr Hematol Oncol 33:565–569. https://doi.org/10.1097/MPH.0b013e3182099a54
Uike N, Miyamura T, Obama K, Takahira H, Sato H, Kozuru M (1993) Parvovirus B19-associated haemophagocytosis in Evans syndrome: aplastic crisis accompanied by severe thrombocytopenia. Br J Haematol 84:530–532
Shirono K, Tsuda H (1995) Parvovirus B19-associated haemophagocytic syndrome in healthy adults. Br J Haematol 89:923–926
Matsumoto Y, Naniwa D, Banno S, Sugiura Y (1998) The efficacy of therapeutic plasmapheresis for the treatment of fatal hemophagocytic syndrome: two case reports. Ther Apher 2:300–304
Chen JS, Lin KH, Lin DT, Chen RL, Jou ST, Su IJ (1998) Longitudinal observation and outcome of nonfamilial childhood haemophagocytic syndrome receiving etoposide-containing regimens. Br J Haematol 103:756–762
Imashuku S, Hibi S, Ohara T, Iwai A, Sako M, Kato M, Arakawa H, Sotomatsu M, Kataoka S, Asami K, Hasegawa D, Kosaka Y, Sano K, Igarashi N, Maruhashi K, Ichimi R, Kawasaki H, Maeda N, Tanizawa A, Arai K, Abe T, Hisakawa H, Miyashita H, Henter JI (1999) Effective control of Epstein-Barr virus-related hemophagocytic lymphohistiocytosis with immunochemotherapy. Histiocyte Society Blood 93:1869–1874
Estlin EJ, Palmer RD, Windebank KP, Lowry MF, Pearson AD (1996) Successful treatment of non-familial haemophagocytic lymphohistiocytosis with interferon and gammaglobulin. Arch Dis Child 75:432–435
Lapphra K, Scheifele D (2011) Can children with minor illnesses be safely immunized? Paediatr Child Health 16:463
Grohskopf LA, Sokolow LZ, Broder KR et al (2017) Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices - United States, 2017-18 influenza season. MMWR Recomm Rep 66:1–20
Acknowledgments
The authors extend particular appreciation to Dr. Jung-Jung Mu and Dr. Sung-Hsi Wei for their experimental help and clinical data. For this type of study, formal consent was not required.
Funding
This study was supported by grants (103-1301-05-05-03, 104-1301-05-05-06, 105-1301-05-05-01, and 106-1301-05-04-02) from the Ministry of Justice, Taiwan. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
Informed consent
The article does not include participants from whom informed consent was required.
Rights and permissions
About this article
Cite this article
Hu, HY., Wei, SY., Huang, WH. et al. Fatal parvovirus B19 infections: a report of two autopsy cases. Int J Legal Med 133, 553–560 (2019). https://doi.org/10.1007/s00414-018-1921-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-018-1921-6