Abstract
Objective
To present long-term data on the Wide Ponto implant bone-anchored hearing system (BAHS) in regards to implant stability, soft tissue reaction and implant loss for two case series undergone either the tissue reduction- or the tissue preservation surgical technique.
Methods
Comparison of two consecutive, prospective case series. Each case series enrolled 24 patients. The case series underwent one-stage implantation of the Wide Ponto implant BAHS using either a linear incision technique with subcutaneous reduction or a linear incision technique without subcutaneous reduction. Implant stability quotient (ISQ) values were measured using resonance frequency analysis and soft tissue reactions were graded according to Holgers’ classification system. Follow-up visits were performed at 10 days, 6 weeks, 6 months, 12 months and annually up to 4 years (tissue preservation) or 5 years (tissue reduction) postoperatively.
Results
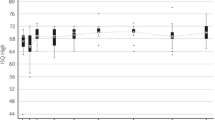
The two case series had homogenous patient populations and followed an identical postoperative scheme. The ISQ values increased consistently the first 12 months for both groups (p ≤ 0.001), and were higher in the tissue preservation case series, (p = 0.04, 9 mm abutment). More than 91% of the soft tissue observations were assessed as Holgers’ grade 0 or 1. One implant (2.1%) was lost due to trauma.
Conclusion
In both case series, the Wide Ponto implant showed increasing implant stability during the follow-up period from the time of surgery, irrespective of surgical technique, indicating good osseointegration. Soft tissue reactions were rare and of minor severity. Implant survival was high.


Similar content being viewed by others
References
Tjellström A, Lindström J, Hallén O, Albrektsson T, Brånemark PI (1981) Osseointegrated titanium implants in the temporal bone. A clinical study on bone-anchored hearing aids. Am J Otol 2:304–310
Holgers KM, Tjellström A, Bjursten LM, Erlandsson BE (1988) Soft tissue reactions around percutaneous implants: a clinical study of soft tissue conditions around skin-penetrating titanium implants for bone-anchored hearing aids. Am J Otol 9:56–59
Dun CAJ, Faber HT, de Wolf MJF, Mylanus EAM, Cremers CWRJ, Hol MKS (2012) Assessment of more than 1,000 implanted percutaneous bone conduction devices: skin reactions and implant survival. Otol Neurotol 33:192–198. https://doi.org/10.1097/MAO.0b013e318241c0bf
Foghsgaard S, Caye-Thomasen P (2014) A new wide-diameter bone-anchored hearing implant-prospective 1-year data on complications, implant stability, and survival. Otol Neurotol 35:1238–1241. https://doi.org/10.1097/MAO.0000000000000345
Wallberg E, Granström G, Tjellström A, Stalfors J (2011) Implant survival rate in bone-anchored hearing aid users: long-term results. J Laryngol Otol 125:1131–1135. https://doi.org/10.1017/S0022215111001447
Reyes RA, Tjellström A, Granström G (2000) Evaluation of implant losses and skin reactions around extraoral bone-anchored implants: a 0- to 8-year follow-up. Otolaryngol Head Neck Surg 122:272–276. https://doi.org/10.1016/S0194-5998(00)70255-5
Kiringoda R, Lustig LR (2013) A meta-analysis of the complications associated with osseointegrated hearing aids. Otol Neurotol 34:790–794. https://doi.org/10.1097/MAO.0b013e318291c651
Granström G (2005) Osseointegration in irradiated cancer patients: an analysis with respect to implant failures. J Oral Maxillofac Surg 63:579–585. https://doi.org/10.1016/j.joms.2005.01.008
Mowinckel MS, Møller MN, Wielandt KN, Foghsgaard S (2016) Clinical outcome of a wide-diameter bone-anchored hearing implant and a surgical technique with tissue preservation. Otol Neurotol 37:374–379. https://doi.org/10.1097/MAO.0000000000000990
Dun CAJ, de Wolf MJF, Hol MKS, Wigren S, Eeg-Olofsson M, Green K et al (2011) Stability, survival, and tolerability of a novel baha implant system: six-month data from a multicenter clinical investigation. Otol Neurotol 32:1001–1007. https://doi.org/10.1097/MAO.0b013e3182267e9c
Nelissen RC, den Besten CA, Faber HT, Dun CAJ, Mylanus EAM, Hol MKS (2016) Loading of osseointegrated implants for bone conduction hearing at 3 weeks: 3-year stability, survival, and tolerability. Eur Arch Otorhinolaryngol 273:1731–1737. https://doi.org/10.1007/s00405-015-3746-y
de Wolf, MJF, Hol MKS, Huygen PLM, Mylanus EAM, Cremers CWRJ (2008) Clinical outcome of the simplified surgical technique for BAHA implantation. Otol Neurotol 29:1100–1108. https://doi.org/10.1097/MAO.0b013e31818599b8
Singam S, Williams R, Saxby C, Houlihan FP (2014) Percutaneous bone-anchored hearing implant surgery without soft-tissue reduction: up to 42 months of follow-up. Otol Neurotol 35:1596–1600. https://doi.org/10.1097/MAO.0000000000000522
Hultcrantz M, Lanis A (2014) A five-year follow-up on the osseointegration of bone-anchored hearing device implantation without tissue reduction. Otol Neurotol 35:1480–1485. https://doi.org/10.1097/MAO.0000000000000352
Høgsbro M, Agger A, Johansen LV (2015) Bone-anchored hearing implant surgery: randomized trial of dermatome versus linear incision without soft tissue reduction–clinical measures. Otol Neurotol 36:805–811. https://doi.org/10.1097/MAO.0000000000000731
Høgsbro M, Agger A, Johansen LV (2017) Successful loading of a bone-anchored hearing implant at 1 week after surgery. Otol Neurotol 38:207–211. https://doi.org/10.1097/MAO.0000000000001312
Strijbos RM, Bom SJH, Zwerver S, Hol MKS (2017) Percutaneous bone-anchored hearing implant surgery: dermatome versus linear incision technique. Eur Arch Otorhinolaryngol 274:109–117. https://doi.org/10.1007/s00405-016-4210-3
van der Pouw, CT, Mylanus EA, Cremers CW (1999) Percutaneous implants in the temporal bone for securing a bone conductor: surgical methods and results. Ann Otol Rhinol Laryngol 108:532–536. https://doi.org/10.1177/000348949910800602
van de Berg, R, Stokroos RJ, Hof JR, Chenault MN (2010) Bone-anchored hearing aid: a comparison of surgical techniques. Otol Neurotol 31:129–135. https://doi.org/10.1097/MAO.0b013e3181c29fec
Hultcrantz M (2011) Outcome of the bone-anchored hearing aid procedure without skin thinning: a prospective clinical trial. Otol Neurotol 32:1134–1139. https://doi.org/10.1097/MAO.0b013e31822a1c47
Calon TGA, van Hoof M, van den Berge H, de Bruijn AJG, van Tongeren J, Hof JR et al (2016) Minimally Invasive Ponto Surgery compared to the linear incision technique without soft tissue reduction for bone conduction hearing implants: study protocol for a randomized controlled trial. Trials 17:540. https://doi.org/10.1186/s13063-016-1662-0
Sardiwalla Y, Jufas N, Morris DP (2017) Direct cost comparison of minimally invasive punch technique versus traditional approaches for percutaneous bone anchored hearing devices. J Otolaryngol Head Neck Surg 46:46. https://doi.org/10.1186/s40463-017-0222-2
Acknowledgements
The authors would like to thank our colleagues Dr. Martin Nue Møller and Prof. Per Cayé-Thomasen and all the nurses and secretaries in the outpatient clinic at the Department of Otorhinolaryngology, Head & Neck Surgery and Audiology, who participated and contributed with their help during all phases of the study. A sincere thank you to Sofia Jonhede and Sara Svensson from Oticon Medical for assisting in the statistical analysis.
Funding
The authors received no funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Reznitsky, M., Wielandt, K. & Foghsgaard, S. Wide diameter bone-anchored hearing system implants: a comparison of long-term follow-up data between tissue reduction and tissue preservation techniques. Eur Arch Otorhinolaryngol 276, 349–356 (2019). https://doi.org/10.1007/s00405-018-5228-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-018-5228-5