Abstract
Introduction
Inhibitors of tumor angiogenesis and metastasis are emerging as important new drug candidates for cancer therapy. Galectin-3 and heparanase have been shown to function in tumor progression and metastatic spread. Both of them exert pleiotropic effects; proliferation, cell migration, differentiation and tissue remodeling. The aim of this study was to investigate heparanase and galectin-3 expression in endometrioid and serous carcinomas of the endometrium and their relation with well-known prognostic factors, in addition to estrogen, progesterone, C-erbB-2, Ki-67 and p53.
Materials and methods
Sixty-four endometrial cancers, which include 24 serous types, were obtained from previously untreated patients. Immunohistochemical analysis of 64 carcinomas, 20 endometrial hyperplasia (ten of simple hyperplasia and ten of complex atypic hyperplasia) and 20 normal endometrium (ten of proliferative and ten of secretory) was performed.
Conclusion
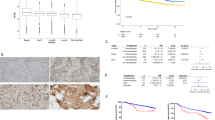
This investigation suggests that the decreased expression of galectin-3 may be involved in the pathogenesis of endometrial carcinomas from normal endometrium to carcinoma. Also down-regulated stromal expression of galectin-3 in endometrial carcinoma may be involved in lymph node metastasis. Further studies on a larger advanced stage (FIGO stage 3–4) endometrial carcinoma group may determine the value of heparanase in the endometrial carcinoma.






Similar content being viewed by others
References
Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A et al (2005) Cancer statistics 2005. CA Cancer J Clin 55:10–30
Sorosky JI (2008) Endometrial cancer. Obstet Gynecol 111:436–447
Ronnett BM, Zaino RJ, Ellenson LH, Kurman JR (2002) Endometrial carcinoma. In: Kurman RJ (ed) Blaustein’s pathology of female genital tract, 5th edn. Springer Verlag, Berlin, pp 501–559
International Federation of Gynecology and Obstetrics: Annual report on the results of treatment in gynecologic cancer: FIGO. Stockholm, Sweden, International Federation of Gynecology and Obstetrics, 1985
Gong HC, Honjo Y, Nangia-Makker P, Hogan V, Mazurak N, Bresalier RS, Raz A (1999) The NH2 terminus of galectin-3 governs cellular compartmentalization and functions in cancer cells. Cancer Res 59:6239–6245
Califice S, Castronovo V, van den Brule F (2004) Galectin-3 and cancer. Int J Oncol 25:983–992
Krzeslak A, Lipinska A (2004) Galectin-3 as a multifunctional protein. Cell Mol Biol Lett 9:305–328
Dangue A, Camby I, Kiss R (2002) Galectins and cancer. Biochim Biophys Acta 1572:285–293
Von Wolff M, Wang X, Gabius HJ, Strowitzki T (2005) Galectin-3 fingerprinting in human endometrium and decidua during the menstrual cycle and in early gestation. Mol Hum Reprod 11:189–194
Lehr JE, Pienta KJ (1998) Preferential adhesion of prostate cancer cells to a human bone marrow endothelial cell line. J Natl Cancer Inst 90:118–123
Robert L, Jacob MP, Fülöp T, Timar J, Hornebeck W (1989) Elastonectin and the elastin receptor. Pathol Biol 37:736–741
Inufusa H, Nakamura M, Adachi T, Aga M, Kurimoto M, Nakatani Y et al (2001) Role of galectin-3 in adenocarcinoma liver metastasis. Int J Oncol 19:913–919
Poirier F (2002) Roles of galectins in vivo. Biochem Soc Symp 69:95–103
Cotter F (2008) Interim phase 2 results on prospect therapeutics’ GCS-100 presented at the 10th international conference on malignant lymphoma. http://www.drugs.com/clinical_trials/interim-phase-2-results-prospect-therapeutics-gcs-100-presented-10th-international-conference-4646.html. Accessed 13 April 2009
Grous JJ, Redfern CH, Mahadevan D, Schindler J (2006) GCS-100, a galectin-3 antagonist, in refractory solid tumors: a phase I study. ASCO Annual Meeting Proceedings http://www.asco.org/ASCO/Abstracts+&+Virtual+Meeting/Abstracts?&vmview=abst_detail_view&confID=40&abstractID=33305. Accessed 22 March 2009
Nakajima M, Irimura T, Nicolson GL (1998) Heparanases and tumor metastasis. J Cell Biochem 36:1–11
Vlodavsky I, Eldor A, Haimovitz-Friedman A, Matzner Y, Ihai-Michaeli R, Lider O et al (1992) Expression of heparanase by platelets and circulating cells of the immune system: possible involvement in diapedesis and extravasation. Invasion Metastasis 12:112–127
Kodama J, Shinyo Y, Hasengaowa, Kusumoto T, Seki N, Nakamura K, Hongo A, Hiramatsu Y (2005) Loss of basement membrane heparan sulfate expression is associated with pelvic lymph node metastasis in invasive cervical cancer. Oncol Rep 14(1):89–92
Liu CJ, Lee PH, Lin DY, Wu CC, Jeng LB, Lin PW et al (2009) Heparanase inhibitor PI-88 as adjuvant therapy for hepatocellular carcinoma after curative resection: a randomized phase II trial for safety, optimal dosage. J Hepatol 50(5):958–968
Parish CR, Freeman C, Brown KJ, Francis DJ, Cowden WB (1999) Identification of sulfated oligosaccharide-based inhibitors of tumor growth and metastasis using novel in vitro assays for angiogenesis and heparanase activity. Cancer Res 59:344–3433
Vlodavsky I, Mohsen M, Lider O, Svahn CM, Ekre HP, Vigoda M et al (1994–1995) Inhibition of tumor metastasis by heparanase inhibiting species of heparin. Invasion Metastasis 14:290–302
Ferro V, Dredge K, Liu L, Hammond E, Bytheway I, Li C et al (2007) PI-88 and novel heparan sulfate mimetics inhibit angiogenesis. Semin Thromb Hemost 33:557–568
Merseburger AS, Kramer MW, Hennenlotter J, Simon P, Knapp J, Hartmann JT et al (2008) Involvement of decreased galectin-3 expression in the pathogenesis and progression of prostate cancer. Prostate 68:72–77
Van den Brule FA, Waltregny D, Liu FT, Castronovo V (2000) Alteration of the cytoplasmic/nuclear expression pattern of galectin-3 correlates with prostate carcinoma progression. Int J Cancer 89:361–367
Shimonishi T, Miyazaki K, Kono N, Sabit H, Tuneyama K, Harada K et al (2001) Expression of endogenous gal-1 and gal-3 in intrahepatic cholangiocarcinoma. Hum Pathol 32:302–310
Sanjuan X, Fernandez PL, Castells A, Catronovo V, Van Den Brule F (1997) Differential expression of galectin 3 and galectin 1 in colorectal cancer progression. Gastroenterology 113:1906–1915
Pacis RA, Pilat MJ, Pienta KJ, Wojno K, Raz A, Hogan V, Cooper CR (2000) Decreased galectin-3 expression in prostate cancer. Prostate 44:118–123
Idikio H (1998) Galectin-3 expression in human breast carcinoma: correlation with cancer histological grade. Int J Oncol 12:1287–1290
Van den Brule FA, Califice S, Castronovo V (2004) Expression of galectins in cancer: a critical review. Glycoconj J 19:537–542
Brustmann H, Riss D, Naude S (2003) Galectin-3 expression in normal, hyperplastic, and neoplastic endometrial tissues. Pathol Res Pract 199:151–158
van den Brule FA, Buicu C, Berchuck A, Bast RC, Deprez M, Liu FT et al (1996) Expression of the 67-kD laminin receptor, galectin-1, and galectin-3 in advanced human uterine adenocarcinoma. Human Pathol 27:1185–1191
Lloyd RV (2001) Distinguishing benign from malignant thyroid lesions: galectin-3 as the latest candidate. Endocr Pathol 12:255–257
Weinberger PM, Adam BL, Gourin CG, Moretz WH, Bollag RJ, Wang BY et al (2007) Association of nuclear, cytoplasmic expression of galectin-3 with beta-catenin/Wnt-pathway activation in thyroid carcinoma. Arch Otolaryngol Head Neck Surg 133:503–510
Carcangiu ML, Steeper T, Zampi G, Rosai J (1985) Anaplastic thyroid carcinoma. A study of 70 cases. Am J Clin Pathol 83:135–158
Langbein S, Brade J, Badawi JK, Hatzinger M, Kaltner H (2007) Gene-expression signature of adhesion/growth-regulatory tissue lectins (galectins) in transitional cell cancer and its prognostic relevance. Histopathology 51:681–690
Yoshimura A, Gemma A, Hosoya Y, Komaki E, Hosomi Y (2003) Increased expression of the LGALS3 (galectin3) gene in human non-small-cell lung cancer. Genes Chromosomes Cancer 37:159–164
Lapis K, Timar J (2002) Role of elastin-matrix interactions in tumor progression. Semin Cancer Biol 12:209–217
Piantelli M, Iacobelli S, Almadori G, Iezzi M, Tinari N, Natoli C et al (2002) Lack of expression of galectin-3 is associated with a poor outcome in node-negative patients with laryngeal squamous-cell carcinoma. J Clin Oncol 20:3850–3856
Plzak J, Betka J, Smetana KJ, Chovanec M, Kaltner H (2004) Galectin-3-an emerging prognostic indicator in advanced head and neck carcinoma. Eur J Cancer 40:2324–2330
Gaudin JC, Arar C, Monsigny M, Legrand A (1997) Modulation of the expression of the rabbit galectin-3 gene by p53 and c-Ha-ras proteins and PMA. Glycobiology 7:1089–1098
Ceehinelli B, Lavra L, Rinaldo C, Iacovelli S, Gurtner A, Gasbarri A et al (2006) Repression of the antiapoptotic molecule galectin-3 by homeodomain-interacting protein kinase 2-activated p53 is required for p53-induced apoptosis. Mol Cell Biol 26:4746–4757
Takenaka Y, Fukumori T, Yoshii T, Oka N, Inohara H, Kim HR, Bresalier RS, Raz A (2004) Nuclear export of phosphorylated galectin-3 regulates its antiapoptotic activity in response to chemotherapeutic drugs. Mol Cell Biol 24:4395–4406
Kim HR, Lin HM, Bilirian H, Raz A (1999) Cell cycle arrest and inhibition of anoikis by galectin-3 in human breast cells. Cancer Res 59:4148–4154
Yang RY, Hsu DK, Liu FT (1996) Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci 93:6737–6742
Moon BK, Lee YJ, Battle P, Jessup JM, Raz A, Kim HRC (2001) Galectin-3 protects human breast carcinoma cells against nitric oxide-induced apoptosis: implication of galectin-3 function during metastasis. Am J Pathol 159:1055–1060
Zubieta MR, Furman D, Barrio M, Bravo AI, Domenichini E, Mordoh J (2006) Galectin-3 expression correlates with apoptosis of tumor-associated lymphocytes in human melanoma biopsies. Am J Pathol 168:1666–1675
van den Brule FA, Engel J, Stetler-Stevenson WG et al (1992) Genes involved in tumor invasion and metastasis are differentially modulated by estradiol and progestin in human breast-cancer cells. Int J Cancer 52:653–657
Le Marer N (2000) Galectin-3 expression in differentiating human myeloid cells. Cell Bio Int 24:245–251
Mackay A, Jones C, Dexter T, Silva RL, Bulmer K, Jones A et al (2003) cDNA microarray analysis of genes associated with ERBB2 (HER2/neu) overexpression in human mammary luminal epithelial cells. Oncogene 22:2680–2688
Petrov SV, Raskin GA, Khasanov RS (2006) A new hypothesis on the role of c-erbB2 oncogene in the progress of breast cancer. Bull Exp Biol Med 142:94–97
Yoshii T, Inohara T, Takenaka Y, Honjo Y, Akahani S, Nomura T, Raz A, Kubo T (2001) Galectin-3 maintains the transformed phenotype of thyroid papillary carcinoma cells. Int J Oncol 18:787–792
Friedmann Y, Vlodavsky I, Aingorn H, Aviv A, Peretz T, Pecker I et al (2000) Expression of heparanase in normal, dysplastic, and neoplastic human colonic mucosa and stroma: evidence for its role in colonic tumourigenesis. Am J Pathol 157:1167–1175
Ikuta M, Podyma KA, Maruyama K, Enomoto S, Yanagishita M (2001) Expression of heparanase in oral cancer cell lines and oral cancer tissues. Oral Oncol 37:177–184
Ueno Y, Yamamoto M, Vlodavsky I, Pecker I, Ohshima K, Fukushima T (2005) Decreased expression of heparanase in glioblastoma multiforme. J Neurosurg 102:513–521
Ikeguchi M, Hirooka Y, Kaibara N (2003) Heparanase gene expression and its correlation with spontaneous apoptosis in hepatocytes of cirrhotic liver carcinoma. Eur J Cancer 39:86–90
Stadlmann S, Moser PL, Pollheimer J, Steiner P, Krugman J, Dirnhofer S, Mikuz G, Margreiter R, Amberger A (2003) Heparanase-1 gene expression in normal, hyperplastic and neoplastic prostatic tissue. Eur J Cancer 39:2229–2233
Watanabe M, Aoki Y, Kase H, Tanaka K (2003) Heparanase expression and angiogenesis in endometrial cancer. Gynecol Obstet Invest 56:77–82
Inamine M, Nagai Y, Hirakawa M, Mekaru K, Yagi C, Masamoto H et al (2008) Heparanase expression in endometrial cancer: analysis of immunohistochemistry. J Obstet Gynaecol 28:634–637
Hasengaowa, Kodama J, Kusumoto T, Seki N, Matsuo T, Ojima Y et al (2006) Heparanase expression in both normal endometrium and endometrial cancer. Int J Gynecol Cancer 16(3):1401–1406
Canaani J, Ilan N, Back S, Gutman G, Vlodavsky I, Grisaru D (2008) Heparanase expression increases throughout the endometrial hyperplasia-cancer sequence. Int J Gynaecol Obstet 101:166–171
Xu X, Rao G, Quiros RM, Kim AW, Miao HQ, Brunn GJ, Platt JL, Gattuso P, Prinz RA (2007) In vivo and in vitro degradation of heparan sulfate (HS) proteoglycans by HPR1 in pancreatic adenocarcinomas. Loss of cell surface HS suppresses fibroblast growth factor 2-mediated cell signaling and proliferation. J Biol Chem 282:2363–2373
El-Assal ON, Yamanoi A, Ono T, Kohno H, Nagasue N (2001) The clinicopathological significance of heparanase and basic fibroblast growth factor expressions in hepatocellular carcinoma. Clin Cancer Res 7:1299–1305
Acknowledgments
This work is supported by grant of ‘BAP’ comission of Pamukkale University, No 2008TPF009. Some of the findings about galectin-3 were presented orally in the 22nd European Pathology Congress and also this presentation was supported in part by grant of ‘BAP’ comission of Pamukkale University, No 2009KKP073 and 2007KRM001. We thank Dr. Mehmet Zencir and Dr. Özgür Önal for their contributions in statistical analysis and Mr. Emin Ateş for technical assistance.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ege, Ç.B., Akbulut, M., Zekioğlu, O. et al. Investigation of galectin-3 and heparanase in endometrioid and serous carcinomas of the endometrium and correlation with known predictors of survival. Arch Gynecol Obstet 284, 1231–1239 (2011). https://doi.org/10.1007/s00404-010-1766-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-010-1766-9