Abstract
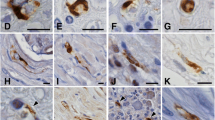
We immunohistochemically examined the brain and peripheral sympathetic ganglia from eight patients with multiple system atrophy (MSA), using an antibody specific for phosphorylated α-synuclein (anti-PSer129). Phosphorylated α-synuclein was deposited in five cellular locations: oligodendroglial cytoplasm and nucleus, and neuronal cytoplasm, processes and nucleus. Many neuronal cytoplasmic inclusions (NCIs) were found in the pontine and inferior olivary nuclei and, to a lesser extent, in the substantia nigra, locus ceruleus, and neocortical and hippocampal neurons. NCIs were also found in the sympathetic ganglia in two out of the eight cases. Moreover, anti-PSer129 immunohistochemistry revealed extensive neuropil pathology; swollen neurites were abundant in the pontine nucleus, delicate neurites were observed in the deeper layers of the cerebral cortex and thalamus, and neuropil threads and dot-like structures were distributed in the basal ganglia and brainstem. Diffuse neuronal cytoplasmic staining (pre-NCI) was frequently found in the pontine and inferior olivary nuclei. Thus, the widespread accumulation of phosphorylated α-synuclein in both glial and neuronal cells is a pathological feature in patients suffering from MSA.



Similar content being viewed by others
References
Bannister R, Oppenheimer DR (1972) Degenerative diseases of the nervous system associated with autonomic failure. Brain 95:457–474
Duda JE, Lee VM, Trojanowski JQ (2000) Neuropathology of synuclein aggregates. J Neurosci Res 61:121–127
Forman MS, Schmidt ML, Kasturi S, Perl DP, Lee VM-Y, Trojanowski JQ (2002) Tau and α-synuclein pathology in amygdala of parkinsonism-dementia complex patients of Guam. Am J Pathol 160:1725–1731
Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg S, Shen J, Takio K, Iwatsubo T (2002) α-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol 4:160–164
Gray F, Vincent D, Hauw JJ (1988) Quantitative study of lateral horn cells in 15 cases of multiple system atrophy. Acta Neuropathol (Berl) 75:513–518
Hamilton RL (2000) Lewy bodies in Alzheimer’s disease: a neuropathological review of 145 cases using α-synuclein immunohistochemistry. Brain Pathol 10:378–384
Hasegawa M, Fujiwara H, Nonaka T, Wakabayashi K, Takahashi H, Lee VM-Y, Trojanowski JQ, Mann D, Iwatsubo T (2002) Phosphorylated α-synuclein is ubiquitinated in α-synucleinopathy lesions. J Biol Chem 277:49071–49076
Hishikawa N, Hashizume Y, Yoshida M, Hirayama M, Sobue G (2002) Neuropathology of pure autonomic failure. Neurol Med (Tokyo) 57:35–43
Hishikawa N, Hashizume Y, Ujihara N, Okada Y, Yoshida M, Sobue G (2003) α-Synuclein-positive structures in association with diffuse neurofibrillary tangles with calcification. Neuropathol Appl Neurobiol 29:280–287
Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, Rohan de Silva HA, Kittel A, Saitoh T (1995) The precursor protein of non-Aβ component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron 14:467–475
Jellinger KA (2003) α-Synuclein pathology in Parkinson’s and Alzheimer’s disease brain: incidence and topographic distribution—a pilot study. Acta Neuropathol 106:191–201
Kahle PJ, Neumann M, Ozmen L, Müller V, Jacobsen H, Spooren W, Fuss B, Mallon B, Macklin WB, Fujiwara H, Hasegawa M, Iwatsubo T, Kretzschmar HA, Haass C (2002) Hyperphosphorylation and insolubility of α-synuclein in transgenic mouse oligodendrocytes. EMBO Rep 3:583–588
Kennedy PGE, Duchen LW (1985) A quantitative study of intermediolateral column cells in motor neuron disease and the Shy-Drager syndrome. J Neurol Neurosurg Psychiatry 48:1103–1106
Konno H, Yamamoto T, Iwasaki Y, Iizuka H (1986) Shy-Drager syndrome and amyotrophic lateral sclerosis. Cytoarchitectonic and morphometric studies of sacral autonomic neurons. J Neurol Sci 73:193–204
Lippa CF, Fujiwara H, Mann DMA, Giasson B, Baba M, Schmidt ML, Nee LE, O’Connell B, Pollen DA, St. George-Hyslop P, Ghetti B, Nochlin D, Bird TD, Cairns NJ, Lee VM-Y, Iwatsubo T, Trojanowski JQ (1998) Lewy bodies contain altered α-synuclein in brains of many familial Alzheimer’s disease patients with mutations in presenilin and amyloid precursor protein genes. Am J Pathol 153:1365–1370
Lippa CF, Schmidt M, Lee VM, Trojanowski JQ (1999) Antibodies to α-synuclein detect Lewy bodies in many Down’s syndrome brains with Alzheimer’s disease. Ann Neurol 45:353–357
Marouteaux L, Campanelli JT, Scheller RH (1988) Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci 8:2804–2815
Mori F, Inenaga C, Yoshimoto M, Umezu H, Tanaka R, Takahashi H, Wakabayashi K (2002) α-Synuclein immunoreactivity in normal and neoplastic Schwann cells. Acta Neuropathol 103:145–151
Mori F, Tanji K, Yoshimoto M, Takahashi H, Wakabayashi K (2002) Demonstration of α-synuclein immunoreactivity in neuronal and glial cytoplasm in normal human brain tissue using proteinase K and fromic acid pretreatment. Exp Neurol 176:98–104
Neumann M, Kahle PJ, Giasson BI, Ozmen L, Borroni E, Spooren W, Müller V, Odoy S, Fujiwara H, Hasegawa M, Iwatsubo T, Trojanowski JQ, Kretzschmar HA, Haass C (2002) Misfolded proteinase K-resistant hyperphosphorylated α-synuclein in aged transgenic mice with locomotor deterioration and in human α-synucleinopathies. J Clin Invest 110:1429–1439
Okochi M, Walter J, Koyama A, Nakajo S, Baba M, Iwatsubo T, Meijer L, Kahle PJ, Haass C (2000) Constitutive phosphorylation of the Parkinson’s disease associated α-synuclein. J Biol Chem 275:390–397
Papp MI, Kahn JE, Lantos PL (1989) Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome). J Neurol Sci 94:79–100
Parkkinen L, Soininen H, Alafuzoff I (2003) Regional distribution of α-synuclein pathology in unimpaired aging and Alzheimer disease. J Neuropathol Exp Neurol 62:363–367
Piao Y-S, Mori F, Hayashi S, Tanji K, Yoshimoto M, Kakita A, Wakabayashi K, Takahashi H (2003) α-Synuclein pathology affecting Bergmann glia of the cerebellum in patients with α-synucleinopathies. Acta Neuropathol 105:403–409
Richter-Landsberg C, Gorath M, Trojanowski JQ, Lee VM-Y (2000) α-Synuclein is developmentally expressed in cultured rat brain oligodendrocytes. J Neurosci Res 62:9–14
Saito Y, Kawashima A, Ruberu NN, Fujiwara H, Koyama S, Sawabe M, Arai T, Nagura H, Yamanouchi H, Hasegawa M, Iwatsubo T, Murayama S (2003) Accumulation of phosphorylated α-synuclein in aging human brain. J Neuropathol Exp Neurol 62:644–654
Sampathu DM, Giasson BI, Pawlyk AC, Trojanowski JQ, Lee VM-Y (2003) Ubiquitination of α-synuclein is not required for formation of pathological inclusions in α-synucleinopathies. Am J Pathol 163:91–100
Takahashi M, Kanuka H, Fujiwara H, Koyama A, Hasegawa M, Miura M, Iwatsubo T (2003) Phosphorylation of α-synuclein characteristic of synucleinopathy lesions is recapitulated in α-synuclein transgenic Dorosophilia. Neurosci Lett 336:155–158
Tanji K, Imaizumi T, Yoshida H, Mori F, Yoshimoto M, Satoh K, Wakabayashi K (2001) Expression of α-synuclein in a human glioma cell line and its up-regulation by interleukin-1β. Neuroreport 12:1909–1912
Tu PH, Galvin JE, Baba M, Giasson B, Tomita T, Leight S, Nakajo S, Iwatsubo T, Trojanowski JQ, Lee VM (1998) Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble α-synuclein. Ann Neurol 44:415–422
Wakabayashi K, Hayashi S, Kakita A, Yamada M, Toyoshima Y, Yoshimoto M, Takahashi H (1998) Accumulation of α-synuclein/NACP is a cytopathological feature common to Lewy body disease and multiple system atrophy. Acta Neuropathol 96:445–452
Wakabayashi K, Yoshimoto M, Fukushima T, Koide R, Horikawa Y, Morita T, Takahashi H (1999) Widespread occurrence of α-synuclein/NACP-immunoreactive neuronal inclusions in juvenile and adult-onset Hallervorden-Spatz disease with Lewy bodies. Neuropathol Appl Neurobiol 25:363–368
Wenning GK, Shlomo YB, Magalhaes M, Daniel SE, Quinn NP (1994) Clinical features and natural history of multiple system atrophy: an analysis of 100 cases. Brain 117:835–845
Yamazaki M, Arai Y, Baba M, Iwatsubo T, Mori O, Katayama Y, Oyanagi K (2000) α-Synuclein inclusions in amygdala in the brains of patients with the parkinsonism-dementia complex of Guam. J Neuropathol Exp Neurol 59:585–591
Yokota O, Terada S, Ishizu H, Tsuchiya K, Kitamura Y, Ikeda K, Ueda K, Kuroda S (2002) NACP/α-synuclein immunoreactivity in diffuse neurofibrillary tangles with calcification (DNCT). Acta Neuropathol 104:333–341
Acknowledgements
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and grants from The Naito Foundation of Japan, The Karoji Memorial Foundation for Medical Research, and The Uehara Memorial Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nishie, M., Mori, F., Fujiwara, H. et al. Accumulation of phosphorylated α-synuclein in the brain and peripheral ganglia of patients with multiple system atrophy. Acta Neuropathol 107, 292–298 (2004). https://doi.org/10.1007/s00401-003-0811-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-003-0811-1