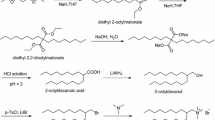
Abstract
Dimeric surfactants, also termed as Gemini surfactants, are regarded as organic materials which have two hydrophilic head groups and two hydrophobic groups in the molecules linked together with a spacer. In this study, dimeric surfactants of quaternary ammonium bromide connected with a trimethylene spacer group (m-3-m) have been investigated as potential micellar solutions for enhanced oil recovery. Static surface tension, interfacial tension as well as optimal salinity characterized their physicochemical and microemulsion properties. Using modeled petroleum fluids, the critical micelle concentration (CMC) was found dependent not only of the chemical architecture of the surfactant but also of the composition in the liquid phase. The nature and/or the length of spacer group participates significantly to the spatial rearrangement of the dimeric surfactants which subsequently altered the surface properties. For the same spacer group, an ultralow interfacial tension was achieved. Encouraging oil solubilization was found for surfactants used with an effect pronounced for longer alkyl chain. Furthermore, both the effects and the presence of metallic divalent ions on the phase behavior were discussed.












Similar content being viewed by others
Notes
Microemulsions are thermodynamically stable, isotropic liquid mixtures of oil, water, and surfactant.
Commercially known as TWEEN 20, it is a form of polyethylene glycolated esterified with a fatty acid.
The compositions of the connate water were modeled from typical reservoir water composition [41].
A quantity representing the strength of the electric field in a solution expressed by \( I=\frac{1}{2}{\displaystyle \sum {c}_i{z}_i^2} \)
References
Sekhon BS (2004) Gemini (dimeric) surfactants. Resonance 9(3):42–49
Yoshimura T, Yoshida H, Ohno A, Esumi K (2003) Physicochemical properties of quaternary ammonium bromide-type trimeric surfactants. J Colloid Interface Sci 267(1):167–172
Diamant H, Andelman D (2003) Models of gemini surfactants. In: Gemini surfactants: interfacial & solution phase behavior. Marcel Dekker, New York, p 16
Zana R (2002) Dimeric and oligomeric surfactants. Behavior at interfaces and in aqueous solution: a review. Adv Colloid Interf Sci 97:205–253
Bunton CA, Robinson L, Schaak J, Stam MF (1971) Catalysis of nucleophilic substitutions by micelles of dicationic detergents. J Org Chem 36(16):2346–2350
Devinsky F, Masarova L, Lacko I (1985) Surface activity and micelle formation of some new bisquaternary ammonium salts. J Colloid Interface Sci 105(1):235–239
Magdassi S, Ben Moshe M, Talmon Y, Danino D (2003) Microemulsions based on anionic gemini surfactant. Colloids Surf A Physicochem Eng Asp 212:1–7
Shukla D, Tyagi VK (2006) Cationic gemini surfactants: a review. J Oleo Sci 55(8):381–390
Bera A, Ojha K, Kumar T, Mandal A (2012) Phase behavior and physicochemical properties of (sodium dodecyl sulfate + brine + propan-1-ol + heptane) microemulsions. J Chem Eng Data 57:1000–1006
Zana R, Benrraou M, Rueff R (1991) Alkanediyl-.alpha., omega.-bis(dimethylalkylammonium bromide) surfactants. 1. Effect of the spacer chain length on the critical micelle concentration and micelle ionization degree. Langmuir 7(6):1072–1075
Villa C, Baldassari S, Chillura Martino DF, Spinella A, Caponetti E (2013) Green synthesis, molecular characterization and associative behavior of some gemini surfactants without a spacer group. Materials 6:1506–1519
Shah DO, Schechter RS (1977) Improved oil recovery by surfactant and polymer flooding. Academic, New York
Santanna VC, Curbelo FDS, Castro Dantas TN, Dantas Neto AA, Albuquerque HS, Garnica AIC (2009) Microemulsion flooding for enhanced oil recovery. J Pet Sci Eng 66:117–120
Winsor PA (1954) Solvent properties of amphiphilic compounds. Butterworths Scientific Publications, London
Kumar P, Mittal KL (1999) Handbook of microemulsion science and technology. Marcel Dekker, New York
Iglauer S, Wu Y, Shuler P, Tang Y, Goddard WA (2010) New surfactant classes for enhanced oil recovery and their tertiary oil recovery potential. J Pet Sci Eng 71:23–29
Jeirani Z, Mohamed Jan B, Si Ali B, Noor IM, See CH, Saphanuchar W (2013) Formulation and phase behavior study of a nonionic triglyceride microemulsion to increase hydrocarbon production. Ind Crop Prod 43(1):15–24
Bertsch H, Rauchalles G (1936) Process for the production of glucosides of higher aliphatic alcohols. Patent No. 2,049,758
Iglauer S, Wu Y, Shuler P, Tang Y, Goddard WA (2009) Alkyl polyglycoside surfactant-alcohol cosolvent formulations for improved oil recovery. Colloids Surf A Physicochem Eng Asp 339:48–59
Iglauer S, Wu Y, Shuler PJ, Blanco M, Y. Tang Y, Goddard WA (2004) Alkyl polyglycoside surfactants for improved oil recovery. In: Symposium on improved oil recovery, SPE–89472–MS
Hughes FA, Lew BW (1970) Physical and functional properties of some higher alkyl polyglucosides. J Am Oil Chem Soc 47(5):162–167
Wu Y, Iglauer S, Shuler P, Tang Y, Goddard WA (2010) Alkyl polyglycoside-sorbitan ester formulations for improved oil recovery. Tenside Surfactant Deterg 47(5):280–287
Green DW, Willhite GP (1998) Enhanced oil recovery. Soc Petrol Eng J. doi:https://doi.org/10.4236/oalib.1101437, Texas
Harkins WD, Brown FE (1918) The determination of surface tension (free surface energy), and the weight if falling drops: the surface tension of wtaer and benzene by the capillary height method. J Am Chem Soc 41(4):499–524
Lando JL, Oakley HT (1967) Tabulated correction factors for the drop-weight-volume determination of surface and interracial tensions. J Colloid Interface Sci 25(4):526–530
Michel JC, Rivière LM, Bellon-Fontaine MN (2001) Measurement of the wettability of organic materials in relation to water content by the capillary rise method. Eur J Soil Sci 52(3):459–467
Mata J, Varade D, Bahadur P (2005) Aggregation behavior of quaternary rsalt based cationic surfactants. Thermochim Acta 428:147–155
Aguila-Hernández J, Trejo A, Gracia-Fadrique J (2001) Surface tension of aqueous solutions of alkanolamines: single amines, blended amines and systems with nonionic surfactants. Fluid Phase Equilib 185:165–175
Iglauer S, Salamah A, Sarmadivaleh M, Liu K, Phan C (2014) Contamination of silica surfaces: impact on water-CO2-quartz and glass contact angle measurements. Int J Greenh Gas Control 22:325–328
Huh C (1979) Interfacial tensions and solubilizing ability of a microemulsion phase that coexists with oil and brine. J Colloid Interface Sci 71(2):408–426
Sheng JJ (2011) Modern chemical enhanced oil recovery: theory and practice. Elsevier, Burlington
Wu Y, Iglauer S, Shuler P, Tang Y, Goddard WA (2010) Branched alkyl alcohol propoxylated sulfate surfactants for improved oil recovery. Tenside Surfactant Deterg 47(3):152–161
Onitsuka E, Beppu J, Yoshimura JT, Koide Y, Shosenji H, Esumi K (2000) Preparation surfactants and surface activity of complexane-type having two and three alkyl chains. J Jpn Oil Chem Soc 49(9):929–973
Daley SJ, Daley RF (2008) Organic chemistry: organic chemistry for the twenty-first century. http://www.ochem4free.info/. Accessed June 2015
Holmberg K, Jönsson B, Kronger B (2003) Surfactants and polymers in aqueous solutions. Wiley, Chichester
Ab Rani MA, Brant A, Crowhurst L, Dolan A, Lui M, Hassan NH, Hallett JP, Hunt PA, Niedermeyer H, Perez-Arlandis JM, Schrems M, Welton T, Wilding R (2011) Understanding the polarity of ionic liquids. Phys Chem Chem Phys 13(37):16831–16840
Davies JT, Rideal EK (1961) Interfacial phenomena. Academic, New York
Zana R (2002) Dimeric (gemini) surfactants: effect of the spacer group on the association behavior in aqueous solution. J Colloid Interface Sci 248(2):203–220
Yoshimura T, Ohno A, Esumi K (2004) Mixed micellar properties of cationic trimeric-type quaternary ammonium salts and anionic sodium n-octyl sulfate surfactants. J Colloid Interface Sci 272(1):191–196
Hua XY, Rosen MJ (1991) Dynamic surface tension of aqueous surfactant solutions. J Colloid Interface Sci 141(1):180–190
Tarek A (2007) Equations of state and PVT analysis. Gulf Publishing Company, Houston
Bansal VK, Shah DO (1978) The effect of divalent cations (Ca++ and Mg++) on the optimal salinity and salt tolerance of petroleum sulfonate and ethoxylated sulfonate mixtures in relation to improved oil recovery. J Am Oil Chem Soc 55(3):67–370
Pine SH (2011) The base-promoted rearrangements of quaternary ammonium salts. Org React 18(4):403–464
Das S, Mondal S, Ghosh S (2013) Physicochemical studies on the micellization of cationic, anionic, and nonionic surfactants in water − polar organic solvent mixtures. J Chem Eng Data 58:2586–2595
Lyon PA, Crow FW, Tomer KB, Gross ML (1984) Analysis of cationic surfactants by mass spectrometry/mass spectrometry with fast atom bombardment. Anal Chem 56(13):2278–2284
Buse J, Badea I, Verrall RE, El-aneed A (2013) A general liquid chromatography tandem mass spectrometry method for the quantitative determination of diquaternary ammonium Gemini surfactant drug delivery agents. J Chromatogr A 1294:98–105
Acknowledgments
The authors extend their gratitude towards Lion Ltd. for synthetizing and kindly supplying the surfactants used in this study. The authors would also like to acknowledge the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT) for the support.
Conflicts of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nguele, R., Sasaki, K., Salim, H.SA. et al. Physicochemical and microemulsion properties of dimeric quaternary ammonium salts with trimethylene spacer for enhanced oil recovery. Colloid Polym Sci 293, 3487–3497 (2015). https://doi.org/10.1007/s00396-015-3701-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-015-3701-x