Abstract
Background
Hyperoxic exposure in vivo (> 95% oxygen) attenuates ischemia-reperfusion injury, but the signaling mechanisms of this cardioprotection are not fully determined. We studied a possible role of nitric oxide (NO) and mitogen activated protein kinases (MAPK) in hyperoxic protection.
Methods
Mice (n = 7–9 in each group) were kept in normoxic or hyperoxic environments for 15 min prior to harvesting the heart and Langendorff perfusion with global ischemia (45 min) and reperfusion (60 min). Endpoints were cardiac function and infarct size. Additional hearts were collected to evaluate MAPK phosphorylation (immunoblot). The nitric oxide synthase inhibitor L-NAME, the ERK1/2 inhibitor PD98059 and the p38 MAPK inhibitor FR167653 were injected intraperitoneally before hyperoxia or normoxia.
Results
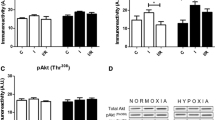
Hyperoxia improved postischemic functional recovery and reduced infarct size (p < 0.05). Hyperoxic exposure caused cardiac phosphorylation of the MAPK family members p38 and ERK1/2, but not JNK. L-NAME, PD98059 and FR167653 all reduced the protection afforded by hyperoxic exposure, but did not influence performance or infarction in hearts of normoxic mice. The hyperoxia-induced phosphorylation of ERK1/2 and p38 was reduced by L-NAME and both MAPK inhibitors.
Conclusion
Nitric oxide triggers hyperoxic protection, and ERK1/2 and p38 MAPK are involved in signaling of protection against ischemia-reperfusion injury.
Similar content being viewed by others
References
Armstrong SC (2004) Protein kinase activation and myocardial ischemia/ reperfusion injury. Cardiovasc Res 61:427–436
Armstrong SC, Delacey M, Ganote CE (1999) Phosphorylation state of hsp27 and p38 MAPK during preconditioning and protein phosphatase inhibitor protection of rabbit cardiomyocytes. J Mol Cell Cardiol 31:555–567
Baines CP, Goto M, Downey JM (1997) Oxygen radicals released during ischemic preconditioning contribute to cardioprotection in the rabbit myocardium. J Mol Cell Cardiol 29:207–216
Barman SA (2005) Effect of nitric oxide on mitogen-activated protein kinases in neonatal pulmonary vascular smooth muscle. Lung 183:325–335
Chang L, Karin M (2001) Mammalian MAP kinase signalling cascades. Nature 410:37–40
Csonka C, Szilvassy Z, Fulop F, Pali T, Blasig IE, Tosaki A, Schulz R, Ferdinandy P (1999) Classic preconditioning decreases the harmful accumulation of nitric oxide during ischemia and reperfusion in rat hearts. Circulation 100:2260–2266
Dawn B, Bolli R (2002) Role of nitric oxide in myocardial preconditioning. Ann N Y Acad Sci 962:18–41
Hiasa G, Hamada M, Ikeda S, Hiwada K (2001) Ischemic preconditioning and lipopolysaccharide attenuate nuclear factor-kappaB activation and gene expression of inflammatory cytokines in the ischemia-reperfused rat heart. Jpn Circ J 65:984–990
Li G, Tokuno S, Tahep ld P, Vaage J, Lowbeer C, Valen G (2001) Preconditioning protects the severely atherosclerotic mouse heart. Ann Thorac Surg 71:1296–1303
Lochner A, Genade S, Hattingh S, Marais E, Huisamen B, Moolman JA (2003) Comparison between ischaemic and anisomycin-induced preconditioning: role of p38 MAPK. Cardiovasc Drugs Ther 17:217–230
Lochner A, Marais E, Du Toit E, Moolman J (2002) Nitric oxide triggers classic ischemic preconditioning. Ann N Y Acad Sci 962:402–414
Lochner A, Marais E, Genade S, Moolman JA (2000) Nitric oxide: a trigger for classic preconditioning? Am J Physiol Heart Circ Physiol 279:H2752–H2765
Maulik N, Sato M, Price BD, Das DK (1998) An essential role of NFkappaB in tyrosine kinase signaling of p38 MAP kinase regulation of myocardial adaptation to ischemia. FEBS Lett 429: 365–369
Maulik N, Watanabe M, Zu YL, Huang CK, Cordis GA, Schley JA, Das DK (1996) Ischemic preconditioning triggers the activation of MAP kinases and MAPKAP kinase 2 in rat hearts. FEBS Lett 396:233–237
Michel MC, Li Y, Heusch G (2001) Mitogen- activated protein kinases in the heart. Naunyn Schmiedebergs Arch Pharmacol 363:245–266
Nakano A, Liu GS, Heusch G, Downey JM, Cohen MV (2000) Exogenous nitric oxide can trigger a preconditioned state through a free radical mechanism, but endogenous nitric oxide is not a trigger of classical ischemic preconditioning. J Mol Cell Cardiol 32:1159–1167
Ping P, Zhang J, Cao X, Li RC, Kong D, Tang XL, Qiu Y, Manchikalapudi S, Auchampach JA, Black RG, Bolli R (1999) PKC-dependent activation of p44/p42 MAPKs during myocardial ischemia-reperfusion in conscious rabbits. Am J Physiol 276:H1468–H1481
Ping P, Takano H, Zhang J, Tang XL, Qiu Y, Li RC, Banerjee S, Dawn B, Balafonova Z, Bolli R (1999) Isoform-selective activation of protein kinase C by nitric oxide in the heart of conscious rabbits: a signaling mechanism for both nitric oxide-induced and ischemia-induced preconditioning. Circ Res 84:587–604
Rakhit RD, Kabir AN, Mockridge JW, Saurin A, Marber MS (2001) Role of G proteins and modulation of p38 MAPK activation in the protection by nitric oxide against ischemia-reoxygenation injury. Biochem Biophys Res Commun 286:995–1002
Ravingerova T, Barancik M, Strniskova M (2003) Mitogen-activated protein kinases: a new therapeutic target in cardiac pathology. Mol Cell Biochem 247:127–138
Roux PP, Blenis J (2004) ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev 68:320–344
Saurin AT, Martin JL, Heads RJ, Foley C, Mockridge JW, Wright MJ, Wang Y, Marber MS (2000) The role of differential activation of p38-mitogen-activated protein kinase in preconditioned ventricular myocytes. Faseb J 14:2237–2246
Steenbergen C (2002) The role of p38 mitogen-activated protein kinase in myocardial ischemia/reperfusion injury; relationship to ischemic preconditioning. Basic Res Cardiol 97:276–285
Zatta AJ, Headrick JP (2005) Mediators of coronary reactive hyperaemia in isolated mouse heart. Br J Pharmacol 144:576–587
Tahepold P, Elfstrom P, Eha I, Kals J, Taal G, Talonpoika A, Valen G, Vaage J, Starkopf J (2002) Exposure of rats to hyperoxia enhances relaxation of isolated aortic rings and reduces infarct size of isolated hearts. Acta Physiol Scand 175:271–277
Tahepold P, Ruusalepp A, Li G, Vaage J, Starkopf J, Valen G (2002) Cardioprotection by breathing hyperoxic gas – relation to oxygen concentration and exposure time in rats and mice. Eur J Cardiothorac Surg 21:987–994
Tahepold P, Vaage J, Starkopf J, Valen G (2003) Hyperoxia elicits myocardial protection through a nuclear factor kappaB-dependent mechanism in the rat heart. J Thorac Cardiovasc Surg 125:650–660
Tanno M, Gorog DA, Bellahcene M, Cao X, Quinlan RA, Marber MS (2003) Tumor necrosis factor-induced protection of the murine heart is independent of p38-MAPK activation. J Mol Cell Cardiol 35:1523–1527
Toma O, Weber NC, Wolter JI, Obal D, Preckel B, Schlack W (2004) Desflurane preconditioning induces time-dependent activation of protein kinase C epsilon and extracellular signal-regulated kinase 1 and 2 in the rat heart in vivo. Anesthesiology 101:1372–1380
Valen G, Tahepold P, Starkopf J, Ruusalepp A, Vaage J (2003) Adaptation to ischemia by hyperoxia – signalling through mitogen activated protein kinases and nuclear factor kappa B. In: Dhalla N HL, Kardani E, Singal PV (eds) Signal Transduction and Cardiac hypertrophy. MA: Kluwer Academic Publications, Boston, pp 461–477
Valen G, Vaage J (2005) Pre- and postconditioning during cardiac surgery. Basic Res Cardiol 100:179–186
Valen G, Yan ZQ, Hansson GK (2001) Nuclear factor kappa-B and the heart. J Am Coll Cardiol 38:307–314
Yada M, Shimamoto A, Hampton CR, Chong AJ, Takayama H, Rothnie CL, Spring DJ, Shimpo H, Yada I, Pohlman TH, Verrier ED (2004) FR167653 diminishes infarct size in a murine model of myocardial ischemia-reperfusion injury. J Thorac Cardiovasc Surg 128:588–594
Yellon DM, Downey JM (2003) Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev 83:1113–1151
Ytrehus K, Liu Y, Downey JM (1994) Preconditioning protects ischemic rabbit heart by protein kinase C activation. Am J Physiol 266:H1145–1152
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ruusalepp, A., Czibik, G., Flatebø, T. et al. Myocardial protection evoked by hyperoxic exposure involves signaling through nitric oxide and mitogen activated protein kinases. Basic Res Cardiol 102, 318–326 (2007). https://doi.org/10.1007/s00395-007-0644-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-007-0644-5