Abstract
Purpose
In our previous study, we showed that Lycium chinense Miller fruit extract (LFE) exerted hepatoprotective effects in mice. In the current study, we examined the effect of LFE on liver enzyme levels in subjects with mild hepatic dysfunction.
Methods
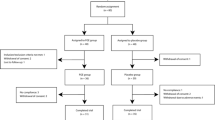
A total of 90 subjects, aged 19 to 70 years old, with abnormal alanine aminotransferase (ALT) levels, were randomly placed into either an LFE (n = 45) treatment group or a placebo group (n = 45). During the 12-week clinical trial, subjects in each group received either LFE or placebo capsules, and were instructed to take four tablets per day (1760 mg/day). The primary outcome of the study was the changes of ALT and γ-glutamyltransferase (GGT) levels in each subject. The safety of LFE supplementation was assessed and adverse events were recorded.
Results
LFE supplementation for 12 weeks resulted in a significant reduction of ALT (P = 0.0498) and GGT (P = 0.0368) levels in comparison to the placebo. No clinically significant changes were observed in any safety parameters.
Conclusion
These results suggest that LFE can be applied to subjects with mild hepatic dysfunction with no possible side effects.
Trial registration
This study was registered at the Clinical Research Information Service (CRIS) as no. KCT0003985.



Similar content being viewed by others
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
Smilin Bell Aseervatham G, Arul Ananth D, Sivasudha T (2018) Chapter 20–The liver: oxidative stress and dietary antioxidants. In: Patel VB, Rajendram R, Preedy VR (eds) The liver. Academic Press, Boston, pp 239–246
Forbes SJ, Newsome PN (2016) Liver regeneration–mechanisms and models to clinical application. Nat Rev Gastroenterol Hepatol 13:473–485. https://doi.org/10.1038/nrgastro.2016.97
Kim HC, Nam CM, Jee SH, Han KH, Oh DK, Suh I (2004) Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ 328:983. https://doi.org/10.1136/bmj.38050.593634.63
West J, Brousil J, Gazis A, Jackson L, Mansell P, Bennett A, Aithal GP (2006) Elevated serum alanine transaminase in patients with type 1 or type 2 diabetes mellitus. QJM 99:871–876
Yanai I, Benjamin H, Shmoish M, Chalifa-Caspi V, Shklar M, Ophir R, Bar-Even A, Horn-Saban S, Safran M, Domany E, Lancet D, Shmueli O (2005) Genome-wide midrange transcription profiles reveal expression level relationships in human tissue specification. Bioinformatics 21:650–659. https://doi.org/10.1093/bioinformatics/bti042
Yang RZ, Park S, Reagan WJ, Goldstein R, Zhong S, Lawton M, Rajamohan F, Qian K, Liu L, Gong DW (2009) Alanine aminotransferase isoenzymes: molecular cloning and quantitative analysis of tissue expression in rats and serum elevation in liver toxicity. Hepatology 49:598–607. https://doi.org/10.1002/hep.22657
Martin-Rodriguez JL, Gonzalez-Cantero J, Gonzalez-Cantero A, Arrebola JP, Gonzalez-Calvin JL (2017) Diagnostic accuracy of serum alanine aminotransferase as biomarker for nonalcoholic fatty liver disease and insulin resistance in healthy subjects, using 3T MR spectroscopy. Medicine (Baltimore) 96:e6770. https://doi.org/10.1097/MD.0000000000006770
Verma S, Jensen D, Hart J, Mohanty SR (2013) Predictive value of ALT levels for non-alcoholic steatohepatitis (NASH) and advanced fibrosis in non-alcoholic fatty liver disease (NAFLD). Liver Int 33:1398–1405. https://doi.org/10.1111/liv.12226
Penn R, Worthington DJ (1983) Is serum gamma-glutamyltransferase a misleading test? Br Med J (Clin Res Ed) 286:531–535. https://doi.org/10.1136/bmj.286.6364.531
Whitfield JB (2001) Gamma glutamyl transferase. Crit Rev Clin Lab Sci 38:263–355. https://doi.org/10.1080/20014091084227
Potterat O (2010) Goji (Lycium barbarum and L. chinense): phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med 76:7–19. https://doi.org/10.1055/s-0029-1186218
Zhu Y-P (1988) Chinese Materia medica: chemistry, pharmacology, and applications. Harwood Academic Publishers, Netherlands
Olatunji OJ, Chen H, Zhou Y (2017) Neuroprotective effect of trans-N-caffeoyltyramine from Lycium chinense against H2O2 induced cytotoxicity in PC12 cells by attenuating oxidative stress. Biomed Pharmacother 93:895–902. https://doi.org/10.1016/j.biopha.2017.07.013
Kim N-H, Baek S-H (2014) Effects of Lycium chinense Miller fruit and its constituent betaine on immunomodulation in Balb/c mice. Korean J Environ Agric 33:189–193. https://doi.org/10.5338/KJEA.2014.33.3.189
Kim MH, Kim EJ, Choi YY, Hong J, Yang WM (2017) Lycium chinense improves post-menopausal obesity via regulation of PPAR-γ and estrogen receptor-alpha/beta expressions. Am J Chin Med 45:269–282. https://doi.org/10.1142/S0192415X17500173
Cui B, Chen Y, Liu S, Wang J, Li S, Wang Q, Li S, Chen M, Lin X (2012) Antitumour activity of Lycium chinensis polysaccharides in liver cancer rats. Int J Biol Macromol 51:314–318. https://doi.org/10.1016/j.ijbiomac.2012.05.004
Zhang R, Kang KA, Piao MJ, Kim KC, Kim AD, Chae S, Park JS, Youn UJ, Hyun JW (2010) Cytoprotective effect of the fruits of Lycium chinense Miller against oxidative stress-induced hepatotoxicity. J Ethnopharmacol 130:299–306. https://doi.org/10.1016/j.jep.2010.05.007
Ahn M, Park JS, Chae S, Kim S, Moon C, Hyun JW, Shin T (2014) Hepatoprotective effects of Lycium chinense Miller fruit and its constituent betaine in CCl4-induced hepatic damage in rats. Acta Histochem 116:1104–1112. https://doi.org/10.1016/j.acthis.2014.05.004
Ha KT, Yoon SJ, Choi DY, Kim DW, Kim JK, Kim CH (2005) Protective effect of Lycium chinense fruit on carbon tetrachloride-induced hepatotoxicity. J Ethnopharmacol 96:529–535. https://doi.org/10.1016/j.jep.2004.09.054
Jung K, Chin YW, Kim YC, Kim J (2005) Potentially hepatoprotective glycolipid constituents of Lycium chinense fruits. Arch Pharm Res 28:1381–1385. https://doi.org/10.1007/BF02977905
Chin YW, Lim SW, Kim SH, Shin DY, Suh YG, Kim YB, Kim YC, Kim J (2003) Hepatoprotective pyrrole derivatives of Lycium chinense fruits. Bioorg Med Chem Lett 13:79–81. https://doi.org/10.1016/s0960-894x(02)00846-6
Kim HP, Lee EJ, Kim YC, Kim J, Kim HK, Park JH, Kim SY, Kim YC (2002) Zeaxanthin dipalmitate from Lycium chinense fruit reduces experimentally induced hepatic fibrosis in rats. Biol Pharm Bull 25:390–392. https://doi.org/10.1248/bpb.25.390
Kim SK, Kim YC, Kim YC (1998) Effects of singly administered betaine on hepatotoxicity of chloroform in mice. Food Chem Toxicol 36:655–661. https://doi.org/10.1016/s0278-6915(98)00024-6
Kim SY, Lee EJ, Kim HP, Kim YC, Moon A, Kim YC (1999) A novel cerebroside from lycii fructus preserves the hepatic glutathione redox system in primary cultures of rat hepatocytes. Biol Pharm Bull 22:873–875. https://doi.org/10.1248/bpb.22.873
Bae U-J, Oh M-R, Park J, Park J-S, Bae E-Y, Chae S-W, Cho BH, Park B-H (2017) Supplementation with Lycium chinense fruit extract attenuates methionine choline-deficient diet-induced steatohepatitis in mice. J Funct Foods 31:1–8. https://doi.org/10.1016/j.jff.2017.01.032
Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F, Beaugrand M, Palau R (2003) Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol 29:1705–1713. https://doi.org/10.1016/j.ultrasmedbio.2003.07.001
Group CHS, Fried MW, Navarro VJ, Afdhal N, Belle SH, Wahed AS, Hawke RL, Doo E, Meyers CM, Reddy KR, Silymarin N (2012) Effect of silymarin (milk thistle) on liver disease in patients with chronic hepatitis C unsuccessfully treated with interferon therapy: a randomized controlled trial. JAMA 308:274–282. https://doi.org/10.1001/jama.2012.8265
Wright R (1979) Liverand biliary disease: pathophysiology, diagnosis, management. Saunders, Philadelphia
Rej R (1978) Aspartate aminotransferase activity and isoenzyme proportions in human liver tissues. Clin Chem 24:1971–1979. https://doi.org/10.1093/clinchem/24.11.1971
Kew MC (2000) Serum aminotransferase concentration as evidence of hepatocellular damage. Lancet 355:591–592. https://doi.org/10.1016/S0140-6736(99)00219-6
Kunutsor SK (2016) Gamma-glutamyltransferase-friend or foe within? Liver Int 36:1723–1734. https://doi.org/10.1111/liv.13221
Lee DH, Blomhoff R, Jacobs DR Jr (2004) Is serum gamma glutamyltransferase a marker of oxidative stress? Free Radic Res 38:535–539. https://doi.org/10.1080/10715760410001694026
Clark JM, Brancati FL, Diehl AM (2003) The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol 98:960–967. https://doi.org/10.1111/j.1572-0241.2003.07486.x
Westerbacka J, Corner A, Tiikkainen M, Tamminen M, Vehkavaara S, Hakkinen AM, Fredriksson J, Yki-Jarvinen H (2004) Women and men have similar amounts of liver and intra-abdominal fat, despite more subcutaneous fat in women: implications for sex differences in markers of cardiovascular risk. Diabetologia 47:1360–1369. https://doi.org/10.1007/s00125-004-1460-1
Group DESIRs, Andre P, Balkau B, Born C, Charles MA, Eschwege E (2006) Three-year increase of gamma-glutamyltransferase level and development of type 2 diabetes in middle-aged men and women: the D.E.S.I.R. cohort. Diabetologia 49:2599–2603. https://doi.org/10.1007/s00125-006-0418-x
Insulin resistance atherosclerosis, Hanley AJ, Williams K, Festa A, Wagenknecht LE, D’Agostino RB Jr, Kempf J, Zinman B, Haffner SM (2004) Elevations in markers of liver injury and risk of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes 53:2623–2632. https://doi.org/10.2337/diabetes.53.10.2623
Lee DH, Silventoinen K, Jacobs DR Jr, Jousilahti P, Tuomileto J (2004) Gamma-Glutamyltransferase, obesity, and the risk of type 2 diabetes: observational cohort study among 20,158 middle-aged men and women. J Clin Endocrinol Metab 89:5410–5414. https://doi.org/10.1210/jc.2004-0505
Perry IJ, Wannamethee SG, Shaper AG (1998) Prospective study of serum gamma-glutamyltransferase and risk of NIDDM. Diabetes Care 21:732–737. https://doi.org/10.2337/diacare.21.5.732
Vozarova B, Stefan N, Lindsay RS, Saremi A, Pratley RE, Bogardus C, Tataranni PA (2002) High alanine aminotransferase is associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Diabetes 51:1889–1895. https://doi.org/10.2337/diabetes.51.6.1889
Reyes-Gordillo K, Shah R, Muriel P (2017) Oxidative stress and inflammation in hepatic diseases: current and future therapy. Oxid Med Cell Longev 2017:3140673. https://doi.org/10.1155/2017/3140673
Sanchez-Valle V, Chavez-Tapia NC, Uribe M, Mendez-Sanchez N (2012) Role of oxidative stress and molecular changes in liver fibrosis: a review. Curr Med Chem 19:4850–4860. https://doi.org/10.2174/092986712803341520
Li S, Tan HY, Wang N, Zhang ZJ, Lao L, Wong CW, Feng Y (2015) The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci 16:26087–26124. https://doi.org/10.3390/ijms161125942
Kim SY, Lee EJ, Kim HP, Lee HS, Kim YC (2000) LCC, a cerebroside from Lycium chinense, protects primary cultured rat hepatocytes exposed to galactosamine. Phytother Res 14:448–451. https://doi.org/10.1002/1099-1573(200009)14:6%3c448::aid-ptr635%3e3.0.co;2-q
Day CR, Kempson SA (2016) Betaine chemistry, roles, and potential use in liver disease. Biochim Biophys Acta 1860:1098–1106. https://doi.org/10.1016/j.bbagen.2016.02.001
Du J, Shen L, Tan Z, Zhang P, Zhao X, Xu Y, Gan M, Yang Q, Ma J, Jiang A, Tang G, Jiang Y, Jin L, Li M, Bai L, Li X, Wang J, Zhang S, Zhu L (2018) Betaine supplementation enhances lipid metabolism and improves insulin resistance in mice fed a high-fat diet. Nutrients 10:131. https://doi.org/10.3390/nu10020131
Kitagawa E, Ota Y, Hasegawa M, Nakagawa T, Hayakawa T (2019) Accumulation of liver lipids induced by vitamin B6 deficiency was effectively ameliorated by choline and to a lesser extent, betaine. J Nutr Sci Vitaminol (Tokyo) 65:94–101. https://doi.org/10.3177/jnsv.65.94
Kwon DY, Jung YS, Kim SJ, Park HK, Park JH, Kim YC (2009) Impaired sulfur-amino acid metabolism and oxidative stress in nonalcoholic fatty liver are alleviated by betaine supplementation in rats. J Nutr 139:63–68. https://doi.org/10.3945/jn.108.094771
Xu L, Huang D, Hu Q, Wu J, Wang Y, Feng J (2015) Betaine alleviates hepatic lipid accumulation via enhancing hepatic lipid export and fatty acid oxidation in rats fed with a high-fat diet. Br J Nutr 113:1835–1843. https://doi.org/10.1017/S0007114515001130
Zhang W, Wang LW, Wang LK, Li X, Zhang H, Luo LP, Song JC, Gong ZJ (2013) Betaine protects against high-fat-diet-induced liver injury by inhibition of high-mobility group box 1 and Toll-like receptor 4 expression in rats. Dig Dis Sci 58:3198–3206. https://doi.org/10.1007/s10620-013-2775-x
Abdelmalek MF, Sanderson SO, Angulo P, Soldevila-Pico C, Liu C, Peter J, Keach J, Cave M, Chen T, McClain CJ, Lindor KD (2009) Betaine for nonalcoholic fatty liver disease: results of a randomized placebo-controlled trial. Hepatology 50:1818–1826. https://doi.org/10.1002/hep.23239
Wang Z, Yao T, Song Z (2010) Extracellular signal-regulated kinases 1/2 suppression aggravates transforming growth factor-beta1 hepatotoxicity: a potential mechanism for liver injury in methionine-choline deficient-diet-fed mice. Exp Biol Med (Maywood) 235:1347–1355. https://doi.org/10.1258/ebm.2010.010160
Liu G, Huang Y, Reis FS, Song D, Ni H (2019) Impact of nutritional and environmental factors on inflammation, oxidative stress, and the microbiome 2019. Biomed Res Int 2019:5716241. https://doi.org/10.1155/2019/5716241
Wang XL, Rainwater DL, VandeBerg JF, Mitchell BD, Mahaney MC (2001) Genetic contributions to plasma total antioxidant activity. Arterioscler Thromb Vasc Biol 21:1190–1195. https://doi.org/10.1161/hq0701.092146
Plaza-Diaz J, Solis-Urra P, Rodriguez-Rodriguez F, Olivares-Arancibia J, Navarro-Oliveros M, Abadia-Molina F, Alvarez-Mercado AI (2020) The gut barrier, intestinal microbiota, and liver disease: molecular mechanisms and strategies to manage. Int J Mol Sci 21:8351. https://doi.org/10.3390/ijms21218351
Soares JB, Pimentel-Nunes P, Roncon-Albuquerque R, Leite-Moreira A (2010) The role of lipopolysaccharide/toll-like receptor 4 signaling in chronic liver diseases. Hepatol Int 4:659–672. https://doi.org/10.1007/s12072-010-9219-x
Acknowledgements
This study was supported by a grant funded by the Cheonyang-gun (Chungnam, Republic of Korea). The authors would like to thank the Writing Center at Jeonbuk National University for its skilled proofreading service.
Author information
Authors and Affiliations
Contributions
MRO, SJJ, SWC, BHP, and SOL conceived the project and designed the protocol; MRO, SJJ, SWC, BHP, and SOL performed the experiments; MRO, BHP, and SOL analyzed the data and wrote the manuscript; BHP and SOL have primary responsibility for the final content of the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oh, MR., Jung, SJ., Chae, SW. et al. Lycium chinense Miller fruit extract lowers liver enzyme levels in subjects with mild hepatic dysfunction: a randomized, double-blind, placebo-controlled clinical trial. Eur J Nutr 62, 1415–1425 (2023). https://doi.org/10.1007/s00394-022-03075-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-022-03075-8