Abstract
Introduction
In this study, we evaluate the efficacy and safety of the biosimilar infliximab, CT-P13, in the treatment of inpatients with severe steroid-refractory colitis.
Methods
A retrospective cohort study of adult colitis patients (UC or isolated Crohn’s colitis) admitted to the University of Chicago inflammatory bowel disease inpatient service between January 2018 and December 2018 for management of severe colitis refractory to IV steroids who received CT-P13 were included in the study. Patients diagnosed with active small bowel Crohn’s disease were excluded. CT-P13 was given as a single infusion of 5 to 10 mg/kg. A comprehensive review of their electronic medical records was performed, and demographic, clinical, laboratory, and endoscopic data were extracted. The primary endpoint was colectomy-free survival.
Results
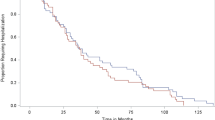
Twenty-one patients with severe steroid-resistant colitis were included. Twelve patients had ulcerative colitis, seven patients had a diagnosis of indeterminate colitis, and two patients had a diagnosis of Crohn’s colitis. The median age was 32.2 years. The median disease duration was 4.3 years, and the median follow-up time was 5.9 months. Patients had a median CRP of 23. All patients had moderate to severe disease on endoscopy. Colectomy-free survival was 76% at 3 months and 70% at 6 months. No severe adverse events were reported in this patient cohort.
Conclusion
A significant proportion of patients with severe colitis failing IV steroids responded to induction therapy with CT-P13. Colectomy-free survival rates were similar to previous randomized trials using originator infliximab as induction therapy in severe steroid-refractory colitis.

Similar content being viewed by others
References
Truelove SC, Witts LJ (1955) Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J 2(4947):1041–1048
Dinesen LC, Walsh AJ, Protic MN, Heap G, Cummings F, Warren BF, George B, Mortensen NJM, Travis SPL (2010) The pattern and outcome of acute severe colitis. J Crohns Colitis 4(4):431–437
Harbord M, Eliakim R, Bettenworth D, Karmiris K, Katsanos K, Kopylov U, Kucharzik T, Molnár T, Raine T, Sebastian S, de Sousa HT, Dignass A, Carbonnel F, for the European Crohn’s and Colitis Organisation [ECCO] (2017) Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: Current Management. J Crohns Colitis 11(7):769–784
Jarnerot G, Hertervig E, Friis-Liby I, Blomquist L, Karlen P, Granno C et al (2005) Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology 128(7):1805–1811
Laharie D, Bourreille A, Branche J, Allez M, Bouhnik Y, Filippi J, Zerbib F, Savoye G, Nachury M, Moreau J, Delchier JC, Cosnes J, Ricart E, Dewit O, Lopez-Sanroman A, Dupas JL, Carbonnel F, Bommelaer G, Coffin B, Roblin X, van Assche G, Esteve M, Färkkilä M, Gisbert JP, Marteau P, Nahon S, de Vos M, Franchimont D, Mary JY, Colombel JF, Lémann M (2012) Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised controlled trial. Lancet 380(9857):1909–1915
Williams JG, Alam MF, Alrubaiy L, Arnott I, Clement C, Cohen D, Gordon JN, Hawthorne AB, Hilton M, Hutchings HA, Jawhari AU, Longo M, Mansfield J, Morgan JM, Rapport F, Seagrove AC, Sebastian S, Shaw I, Travis SPL, Watkins A (2016) Infliximab versus ciclosporin for steroid-resistant acute severe ulcerative colitis (CONSTRUCT): a mixed methods, open-label, pragmatic randomised trial. Lancet Gastroenterol Hepatol 1(1):15–24
Sjoberg M, Walch A, Meshkat M, Gustavsson A, Jarnerot G, Vogelsang H et al (2012) Infliximab or cyclosporine as rescue therapy in hospitalized patients with steroid-refractory ulcerative colitis: a retrospective observational study. Inflamm Bowel Dis 18(2):212–218
van der Valk ME, Mangen MJ, Leenders M, Dijkstra G, van Bodegraven AA, Fidder HH et al (2014) Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFalpha therapy: results from the COIN study. Gut 63(1):72–79
Braun J, Kudrin A (2016) Switching to biosimilar infliximab (CT-P13): Evidence of clinical safety, effectiveness and impact on public health. Biologicals 44(4):257–266
European Medicines Agency (2017) Biosimilars in the EU- Information guide for healthcare professionals. Prepared jointly by the European Medicines Agency and the European Commission. [Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Leaflet/2017/05/WC500226648.pdf
FDA (2017) FDA’s Overview of the Regulatory Guidance for the Development and Approval of Biosimilar Products in the US. [Available from: https://www.fda.gov/downloads/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/therapeuticbiologicapplications/biosimilars/ucm428732.pdf
Ben-Horin S, Vande Casteele N, Schreiber S, Lakatos PL (2016) Biosimilars in inflammatory bowel disease: facts and fears of extrapolation. Clin Gastroenterol Hepatol 14(12):1685–1696
Feagan BG, Choquette D, Ghosh S, Gladman DD, Ho V, Meibohm B, Zou G, Xu Z, Shankar G, Sealey DC, Russell AS (2014) The challenge of indication extrapolation for infliximab biosimilars. Biologicals 42(4):177–183
Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR et al (2005) Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 19 Suppl A:5A–36A
Jorgensen KK, Olsen IC, Goll GL, Lorentzen M, Bolstad N, Haavardsholm EA et al (2017) Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet 389(10086):2304–2316
Jahnsen J, Detlie TE, Vatn S, Ricanek P (2015) Biosimilar infliximab (CT-P13) in the treatment of inflammatory bowel disease: a Norwegian observational study. Expert Rev Gastroenterol Hepatol 9(Suppl 1):45–52
Kaniewska M, Moniuszko A, Rydzewska G (2017) The efficacy and safety of the biosimilar product (Inflectra((R))) compared to the reference drug (Remicade((R))) in rescue therapy in adult patients with ulcerative colitis. Prz Gastroenterol 12(3):169–174
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
AS has received funding from AbbVie, Celltrion, and Takeda. SD has received grant support from Pfizer. JP has received grants from Takeda and Abbvie and serves as a consultant for Verastem. He was on the advisory board for Pfizer and Janssen. DTR is a consultant and has received grant support from Abbvie, Merck & Co., Janssen, Takeda, and Pfizer. RDC is a consultant at Abbvie, Celgene, Janssen, Pfizer, Takeda, and UCB Pharma.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ollech, J.E., Normatov, I., Peleg, N. et al. Efficacy of biosimilar infliximab CT-P13 among inpatients with severe steroid-refractory colitis. Int J Colorectal Dis 35, 2113–2116 (2020). https://doi.org/10.1007/s00384-020-03703-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-020-03703-x