Abstract
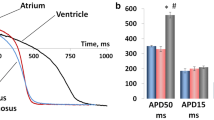
We assessed the functional properties in atrial and ventricular myocardium (using isolated cardiac strips) of smooth dogfish (Mustelus canis), clearnose skate (Raja eglanteria), and sandbar shark (Carcharhinus plumbeus) by blocking Ca2+ release from the sarcoplasmic reticulum (SR) with ryanodine and thapsigargin and measuring the resultant changes in contraction–relaxation parameters and the force–frequency relationship at 20 °C and 30 °C. We also examined ultrastructural differences with electron microscopy. In tissues from smooth dogfish, net force (per cross-sectional area) and measures of the speeds of contraction and relaxation were all higher in atrial than ventricular myocardium at both temperatures. Atrial-ventricular differences were evident in the other two species primarily in measures of the rates of contraction and relaxation. Ryanodine-thapsigargin treatment reduced net force and its maximum positive first derivative (i.e., contractility), and increased time to 50 % relaxation in atrial tissue from smooth dogfish at 30 °C. It also increased times to peak force and half relaxation in clearnose skate atrial and ventricular tissue at both temperatures, but only in atrial tissue from sandbar shark at 30 °C; indicating that SR involvement in excitation–contraction (EC) coupling is species- and temperature-specific in elasmobranch fishes, as it is in teleost fishes. Atrial and ventricular myocardium from all three species displayed a negative force–frequency relationship, but there was no evidence that SR involvement in EC coupling was influenced by heart rate. SR was evident in electron micrographs, generally located in proximity to mitochondria and intercalated discs, and to a lesser extent between the myofibrils; with mitochondria being more numerous in ventricular than atrial myocardium in all three species.











Similar content being viewed by others
Abbreviations
- CICR:
-
Ca2+-induced Ca2+-release
- +dF/dt max :
-
Maximum rate of force development (mN mm−2 s−1)
- −dF/dt min :
-
Maximum rate of relaxation (mN mm−2 s−1)
- DMSO:
-
Dimethyl sulfoxide
- EC:
-
Excitation–contraction
- ECG:
-
Electrocardiogram
- G:
-
Glycogen storage granules
- h:
-
Hours
- Hz:
-
Hertz
- ICD:
-
Intercalated discs
- kHz:
-
Kilohertz
- M:
-
Mitochondria
- min:
-
Minutes
- mm:
-
Millimeter
- mN:
-
MilliNewtons
- ms:
-
Millisecond
- MHC:
-
Myosin heavy chains
- NCX:
-
Na+–Ca2+ exchanger
- nm:
-
Nanometers
- P:
-
Probability
- s:
-
Seconds
- SL:
-
Sarcolemma
- SERCA:
-
SR Ca2+-ATPase
- SR:
-
Sarcoplasmic reticulum
- Z:
-
Z-line
References
Able KW, Fahay MP (2010) Ecology of the estuarine fishes: temperate waters of the western North Atlantic. The John Hopkins University Press, Baltimore
Aho E, Vornanen M (1998) Ca2+-ATPase activity and Ca2+ uptake by sarcoplasmic reticulum in fish heart: effects of thermal acclimation. J Exp Biol 201:525–532
Aho E, Vornanen M (1999) Contractile properties of atrial and ventricular myocardium of the heart of rainbow trout Oncorhynchus mykiss: effects of thermal acclimation. J Exp Biol 202:2663–2677
Asgrimsson H, Johansson M, Arnardottir SA (1995) Excitation and contraction in atrial and ventricular myocardium of the guinea-pig. Acta Physiol Scand 153:133–141
Baumann H, Wallace RB, Tagliaferri T, Gobler CJ (2015) Large natural pH, CO2 and O2 fluctuations in a temperate tidal salt marsh on diel, seasonal, and interannual time scales. Estuaries Coasts 38:220–231
Bers DM (2002) Cardiac excitation–contraction coupling. Nature 415:198–205
Bootman MD, Higazi DR, Coombes S, Roderick HL (2006) Calcium signaling during excitation–contraction coupling in mammalian atrial myocytes. J Cell Sci 119:3915–3925
Bottinelli R, Canepari M, Cappelli V, Reggiani C (1995a) Maximum speed of shortening and ATPase activity in atrial and ventricular myocardia of hyperthyroid rats. Am J Physiol 269:C785–C790
Bottinelli R, Canepari M, Pellegrino M, Reggiani C (1995b) Force–velocity properties of human skeletal muscle fibres: myosin heavy chain isoform and temperature dependence. J Physiol 495:573–586
Breitburg DL, Hondorp D, Audemard C, Carnegie RB, Burrell RB, Trice M, Clark V (2015a) Landscape-level variation in disease susceptibility related to shallow-water hypoxia. PLoS One 10:1–27
Breitburg DL, Salisbury J, Bernhard JM, Cai W-J, Dupont S, Doney SC, Kroeker KJ, Levin LA, Long WC, Milke LM, Miller SH, Phelan B, Passow U, Seibel BA, Todgham AR, Tarrant AM (2015b) And on top of all that… Coping with ocean acidification in the midst of many stressors. Oceanography 28:48–461
Brill RW, Lai NC (2016) Elasmobranch cardiovascular system. In: Shadwick RE, Farrell AP, Brauner CJ (eds) Fish physiology, vol 34B., Physiology of elasmobranch fishes—internal processesAcademic Press, San Diego, pp 2–83
Collette BB, Klein-MacPhee G (2002) Bigelow and Schroeder’s fishes of the Gulf of Maine, 3rd edn. Smithsonian Institution Press, Washington, DC
Compagno LJV (1984) FAO species catalogue. Sharks of the world. An annotated and illustrated catalogue of shark species known to date, vol 4, part 2—Carcharhiniformes, FAO Fish Synop 125(4/2). FAO, Rome, pp 251–655
Compagno LJV (1990) Alternative life-history styles of cartilaginous fishes in time and space. Environ Biol Fish 28:33–75
Conrath CL, Musick JA (2008) Investigations into depth and temperature habitat utilization and overwintering grounds of juvenile sandbar sharks, Carcharhinus plumbeus: the importance of near shore North Carolina waters. Environ Biol Fish 82:123–131
Cooper AR, Morris S (2004) Haemoglobin function and respiratory status of the Port Jackson shark, Heterodontus portusjacksoni, in response to lowered salinity. J Comp Physiol B 174:223–236
Cotter PA, Han AJ, Everson JJ, Rodnick KJ (2008) Cardiac hemodynamics of the rainbow trout (Oncorhynchus mykiss) using simultaneous Doppler echocardiography and electrocardiography. J Exp Zool 309A:243–254
Cros C, Sallé L, Warren DE, Shiels HA, Brette F (2014) The calcium stored in the sarcoplasmic reticulum acts as a safety mechanism in rainbow trout heart. Am J Physiol 307:R1493–R1501
Davie PS, Farrell AP (1991) Cardiac performance of an isolated heart preparation from the dogfish (Squalus acanthias): the effects of hypoxia and coronary artery perfusion. Can J Zool 69:1822–1828
Davie PS, Franklin CE (1992) Myocardial oxygen consumption and mechanical efficiency of a perfused dogfish heart preparation. J Comp Physiol 162:256–262
Dowd W, Brill R, Bushnell P, Musick J (2006) Standard and routine metabolic rates of juvenile sandbar sharks (Carcharhinus plumbeus Nardo), including the effects of body mass and acute temperature change. Fish Bull 104:323–331
Driedzic WR, Gesser H (1988) Differences in force–frequency relationships and calcium dependency between elasmobranch and teleost hearts. J Exp Biol 140:227–241
Farrell AP (1991) From hagfish to tuna: a perspective on cardiac-function in fish. Physiol Zool 64:1137–1164
Farrell AP, Jones DR (1992) The heart. In: Hoar WS, Randall DJ, Farrell AP (eds) Fish physiology, vol 12A., The cardiovascular systemAcademic Press, San Diego, pp 1–87
Galli GLJ (2006) The role of the sarcoplasmic reticulum in the generation of high heart rates and blood pressures in reptiles. J Exp Biol 209:1956–1963
Galli GJ, Shiels H (2012) The sarcoplasmic reticulum in the vertebrate heart. In: Sedmera D, Wang T (eds) Ontogeny and phylogeny of the vertebrate heart. Springer, New York, pp 103–124
Galli GJ, Gesser H, Taylor EW, Shiels HA, Wang T (2006) The role of the sarcoplasmic reticulum in the generation of high heart rates and blood pressures in reptiles. J Exp Biol 209:1956–1963
Galli G, Shiels H, Brill R (2009) Cardiac temperature sensitivity in yellowfin tuna (Thunnus albacares), bigeye tuna (T. obesus), mahimahi (Coryphaena hippurus) and swordfish (Xiphias gladius). Physiol Biochem Zool 82:280–290
Gamperl AK, Shiels HA (2014) Cardiovascular system. In: Evans DH, Claiborne JB, Currie S (eds) The physiology of fishes, 4th edn. CRC Press, Boca Raton, pp 33–79
Genge C, Hove-Madsen L, Tibbits GF (2012) Functional and structural differences in atria versus ventricles in teleost hearts. In: Turker H (ed) New advances and contributions to fish biology. INTECH Open Access Publisher, pp 221–245
Genge CE, Davidson WS, Tibbits GF (2013) Adult teleost heart expresses two distinct troponin C paralogs: cardiac TnC and a novel and teleost-specific ssTnC in a chamber- and temperature-dependent manner. Physiol Genomics 45:866–875
Gillis TE, Klaiman JM (2011) The influence of PKA treatment on the Ca2+ activation of force generation by trout cardiac muscle. J Exp Biol 214:1989–1996
Gillis TE, Tibbits GF (2002) Beating the cold, the functional evolution of cardiac troponin C in teleost fish. Comp Biochem Physiol 132:763–772
Gillis TE, Marshall CR, Xue X-H, Borgford TJ, Tibbits GF (2000) Ca2+ binding to cardiac troponin C: effects of temperature and pH on mammalian and salmonid isoforms. Am J Physiol 279:R1707–R1715
Grubbs RD, Musick JA (2007) Spatial delineation of summer nursery areas for juvenile sandbar sharks in Chesapeake Bay, Virginia. Am Fish Soc Symp 50:63–86
Grubbs RD, Musick JA, Conrath CL, Romaine JG (2007) Long-term movements, migration, and temporal delineation of summer nurseries for juvenile sandbar sharks in the Chesapeake Bay region. Am Fish Soc Symp 50:87–108
Haverinen J, Vornanen M (2009) Comparison of sarcoplasmic reticulum calcium content in arterial and ventricular myocytes of three fish species. Am J Physiol 297:R1180–R1187
Hofmann GE, Smith JE, Johnson KS, Send U, Levin LA, Micheli F, Paytan A, Price NN, Peterson B, Takeshita Y, Matson PG, Derse Crook E, Kroeker KJ, Cristina Gambi M, Rivest EB, Frieder CA, Yu PC, Martz TR (2011) High-frequency dynamics of ocean pH: a multi-ecosystem comparison. PLoS One 6:1–11
Hove-Madsen L (1992) The influence of temperature on ryanodine sensitivity and the force–frequency relationship in the myocardium of the rainbow trout. J Exp Biol 167:47–60
Hove-Madsen L, Llach L, Tort L (1998) Quantification of Ca 2+ uptake in the sarcoplasmic reticulum of trout ventricular myocytes. Am J Physiol 275:R2070–R2080
Hove-Madsen L, Llach A, Tort L (1999) Quantification of calcium release from the sarcoplasmic reticulum in rainbow trout atrial myocytes. Pflügers Arch 438:545–552
Hove-Madsen L, Llach L, Tort L (2001) The function of the sarcoplasmic reticulum is not inhibited by low temperatures in trout atrial myocytes. Am J Physiol 281:R1902–R1906
Karasinski J (1993) Diversity of native myosin and myosin heavy chain in fish skeletal muscles. Comp Biochem Physiol 106B:1041–1047
Karasinski J, Sokalski A, Kilarski W (2001) Correlation of myofibrillar ATPase activity and myosin heavy chain content in ventricular and atrial myocardium of fish heart. Folia Histochem Cytobiol 39:23–28
Keen JE, Farrell AP, Tibbits GF, Brill RW (1992) Cardiac dynamics in tunas. II. Effect of ryanodine, calcium, and adrenaline on force–frequency relationships in atrial strips from skipjack tuna, Katsuwonus pelamis. Can J Zool 70:1211–1217
Keen JE, Vianzon D-M, Farrell AP, Tibbits GF (1994) Effect of temperature and temperature acclimation on the ryanodine sensitivity of the trout myocardium. J Comp Physiol 164B:438–443
Kiraly SJ, Moore JA, Jasinski PA (2003) Deepwater and other sharks of the U.S. Atlantic Ocean exclusive economic zone. Mar Fish Rev 65:1–20
Klimley AP (2013) The Biology of Sharks and Rays. The University of Chicago Press, Chicago
Korajoki H, Vornanen M (2009) Expression of calsequestrin in atrial and ventricular muscle of thermally acclimated rainbow trout. J Exp Biol 212:3403–3414
Korajoki H, Vornanen M (2012) Expression of SERCA and phospholamban in rainbow trout (Oncorhynchus mykiss) heart: comparison of atrial and ventricular tissue and effects of thermal acclimation. J Exp Biol 215:1162–1169
Korajoki H, Vornanen M (2014) Species- and chamber-specific responses of 12 kDa FK506-binding protein to temperature in fish heart. Fish Physiol Biochem 40:539–549
Lai NC, Graham JB, Bhargava V, Shabetai R (1996) Mechanisms of venous return and ventricular filling in elasmobranch fish. Am J Physiol 270:H1766–H1771
Lai NC, Graham JB, Dalton N, Shabetai R, Bhargava V (1998) Echocardiographic and hemodynamic determinations of the ventricular filling pattern in some teleost fishes. Physiol Zool 71:157–167
Lai NC, Dalton N, Lai YY, Kwong C, Rasmussen R, Holts D, Graham JB (2004) A comparative echocardiographic assessment of ventricular function in five species of sharks. Comp Biochem Physiol 137A:505–521
Lo HM, Lin FY, Lin JL, Hsu KL, Chiang FT, Tseng CD, Tseng YZ (1999) Impaired cardiac performance relating to delayed left atrial activation after atrial compartment operation for chronic atrial fibrillation. Pacing Clin Electrophysiol 22:379–381
Luft JH (1977) Improvements in epoxy resin embedding methods. J Biophys Biochem Cy 9:409–414
Marshall H, Field L, Afiadata A, Sepulveda C, Skomal G, Bernal D (2012) Hematological indicators of stress in longline captured sharks. Comp Biochem Physiol 162:121–129
Maylie J, Morad M (1979) Inotropic effects of adrenaline in dogfish heart (Squalus acanthias). Bull Mt Desert Isl Biol Lab 19:87–92
Maylie J, Morad M (1981) Ionic and pharmacological characterization of excitation and contraction in dogfish (Squalus acanthias). Bull Mt Desert Isl Biol Lab 20:122–126
Maylie J, Morad M (1995) Evaluation of T- and L-type Ca2+ currents in shark ventricular myocytes. Am J Physiol 269:H1695–H1703
Maylie J, Nunzi MG, Morad M (1979) Excitation–contraction coupling in ventricular muscle of dogfish (Squalus acanthias). Bull Mt Desert Isl Biol Lab 19:84–87
Maylie J, Varnum M, Morad M (1994) Characterization of a voltage-gated cardiac potassium channel in Squalus acanthias and its expression in Xenopus oocytes injected with mRNA isolated from hearts of Squalus acanthias. Bull Mt Desert Isl Biol Lab 33:29–30
McCandless CT, Pratt HL, Kohler NE, Merson RR, Recksiek CW (2007) Distributions, localized abundance, movements and migrations of juvenile sandbar sharks tagged in Delaware Bay. Am Fish Soc Symp 50:45–62
McEachran JD, Musick JA (1975) Distribution and relative abundance of seven species of skates (Pisces: Rajidae) which occur between Nova Scotia and Cape Hatteras. Fish Bull 73:110–136
Medved RJ, Marshall JA (1981) Feeding behavior and biology of young sandbar sharks, Carcharhinus plumbeus (Pisces, Carcharhinidae), in Chincoteague Bay, Virginia. Fish Bull 79:441–447
Medved RJ, Stillwell CE, Casey JG (1985) Stomach contents of young sandbar sharks, Carcharhinus plumbeus, in Chincoteague Bay, Virginia. Fish Bull 83:395–402
Methling C, Steffensen JF, Skov PV (2012) The temperature challenges on cardiac performance in winter-quiescent and migration-stage eels Anguilla anguilla. Comp Biochem Physiol 163A:66–73
Näbauer M, Morad M (1992) Modulation of contraction by intracellular Na+ via Na+–Ca2+ exchange in single shark (Squalus acanthias) ventricular myocytes. J Physiol 457:627–637
Olson KR, Farrell AP (2006) The Cardiovascular System. In: Evans DH, Claiborne JB (eds) The physiology of fishes. CRC Press, Boca Raton, pp 119–152
Prosser CL (1973) Chapter 9, temperature. In: Prosser CL (ed) Comparative animal physiology. W.B. Saunders Company, Philadelphia, pp 317–361
Reum JCP, Alin SR, Feely RA, Newton J, Warner M, McElhany P (2014) Seasonal carbonate chemistry covariation with temperature, oxygen, and salinity in a fjord estuary: implications for the design of ocean acidification experiments. PLoS One 9:1–12
Rousseau E, Smith JS, Meissner G (1987) Ryanodine modifies conductance and gating behavior of single Ca2+ release channel. Am J Physiol 253:C364–C368
Sætersdal TS, Justesen N-P, Krohnstad AW (1974) Ultrastructure and innervation of the teleostean atrium. J Mol Cell Cardiol 6:415–437
Sagara Y, Inesi G (1991) Inhibition of the sarcoplasmic reticulum Ca2+ transport ATPase by thapsigargin at subnanomolar concentrations. J Biol Chem 266:13503–13506
Sanchez-Quintana D, Hurle J (1987) Ventricular myocardial architecture in marine fishes. Anat Rec 217:263–273
Santer RM (1974) The organization of the sarcoplasmic reticulum in teleost ventricular myocardial cells. Cell Tissue Res 151:395–402
Schwartz FJ (1996) Biology of the clearnose skate, Raja eglanteria, from North Carolina. Fla Sci 59:82–95
Shiels HA, Farrell AP (1997) The effect of temperature and adrenaline on the relative importance of the sarcoplasmic reticulum in contributing Ca2+ to force development in isolated ventricular trabeculae from rainbow trout. J Exp Biol 200:1607–1621
Shiels HA, Galli GL (2014) The sarcoplasmic reticulum and the evolution of the vertebrate heart. Physiology 29:456–469
Shiels HA, Sitsapesan R (2015) Is there something fishy about the regulation of the ryanodine receptor in the fish heart? Exp Physiol 100:1412–1420
Shiels HA, White E (2005) Temporal and spatial properties of cellular Ca2+ flux in trout ventricular myocytes. Am J Physiol 288:R1756–R1766
Shiels HA, Freund EV, Farrell AP, Block BA (1999) The sarcoplasmic reticulum plays a major role in isometric contraction in atrial muscle of yellowfin tuna. J Exp Biol 202:881–890
Shiels HA, Vornanen M, Farrell AP (2002a) Temperature dependence of cardiac sarcoplasmic reticulum function in rainbow trout myocytes. J Exp Biol 205:3631–3639
Shiels HA, Vornanen M, Farrell AP (2002b) The force–frequency relationship in fish hearts—a review. Comp Biochem Physiol 132A:811–826
Shiels HA, Di Maio A, Thompson S, Block BA (2011) Warm fish with cold hearts: thermal plasticity of excitation–contraction coupling in bluefin tuna. Proc Biol Sci 278:18–27
Stillwell CE, Kohler NE (1993) Food habits of the sandbar shark Carcharhinus plumbeus off the U.S. northeast coast, with estimates of daily ration. Fish Bull 91:138–150
Thomas MJ, Hamman BN, Tibbits GF (1996) Dihydropyridine and ryanodine binding in ventricles from rat, trout, dogfish, and hagfish. J Exp Biol 199:1999–2009
Thompson AP, O’Shea JE (1997) The unusual adrenergic-like excitatory action of acetylcholine on the ventricular cardiac muscle of the horned shark, Heterodontus portusjacksoni. Physiol Zool 70:135–142
Tibbits GF, Hove-Madsen L, Bers DM (1991) Calcium-transport and the regulation of cardiac contractility in teleosts: a comparison with higher vertebrates. Can J Zool 69:2014–2019
Tibbits GF, Moyes CD, Hove-Madsen L (1992) Excitation–contraction coupling in the teleost heart. In: Hoar WS, Randall DJ, Farrell AP (eds) Fish physiology, vol 12., The cardiovascular systemAcademic Press, San Diego, pp 267–304
Vornanen M (1994) Seasonal adaptation of crucian carp (Carassius carassius L.) heart: glycogen stores and lactate dehydrogenase activity. Can J Zool 72:433–442
Vornanen M, Haverinen J (2013) A significant role of sarcoplasmic reticulum in cardiac contraction of a basal vertebrate, the river lamprey (Lampetra fluviatilis). Acta Physiol 207:269–279
Vornanen M, Tuomennoro J (1999) Effects of acute anoxia on heart function in crucian carp (Carassius carassius L.) heart: importance of cholinergic and purinergic control. Am J Physiol 277:R465–R475
Vornanen M, Ryӧkkynen A, Nurmi A (2002a) Temperature dependent expression of sarcolemmal K+ currents in rainbow trout atrial and ventricular myocytes. Am J Physiol 282:R1191–R1199
Vornanen M, Shiels HA, Farrell AP (2002b) Plasticity of excitation–contraction coupling in fish cardiac myocytes. Comp Biochem Physiol 132A:827–846
Walden AP, Dibb KM, Trafford AW (2009) Differences in intracellular calcium homeostasis between atrial and ventricular myocytes. J Mol Cell Cardiol 46:463–473
Weissgerber TL, Milic NM, Winham SJ, Garovic VN (2015) Beyond bar and line graphs: time for a new data presentation paradigm. PLoS Biol 13:1–10
Yelon D, Horne S, Stainier D (1999) Restricted expression of cardiac myosin genes reveals regulated aspects of heart tube assembly in zebrafish. Dev Biol 214:23–37
Acknowledgments
The authors gratefully acknowledge Holly Shiels and Gina Galli (University of Manchester, U.K.) for their critical reviews of earlier versions of this manuscript, although any errors of omission or commission are solely ours. We also greatly recognized the entire staff of the VIMS Eastern Shore Laboratory for their ongoing hospitality and help in running the experiments. Support was provided by the Core Facility for Integrated Microscopy, University of Copenhagen; the University of Indiana South Bend; and the Northeast Fisheries Science Center, National Marine Fisheries Service, National Oceanic and Atmospheric Administration (NOAA). This is contribution 3582 from the Virginia Institute of Marine Science, College of William and Mary. The opinions expressed herein are those of the authors and do not necessarily reflect the views of the US Department of Commerce, National Oceanic and Atmospheric Administration (NOAA), or any of their sub-agencies. Likewise, mention of trade names, products, or commercial companies is for identification purposes only and does not imply endorsement by NOAA or any of its sub-agencies. The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H.V. Carey.
Rights and permissions
About this article
Cite this article
Larsen, J., Bushnell, P., Steffensen, J. et al. Characterization of the functional and anatomical differences in the atrial and ventricular myocardium from three species of elasmobranch fishes: smooth dogfish (Mustelus canis), sandbar shark (Carcharhinus plumbeus), and clearnose skate (Raja eglanteria). J Comp Physiol B 187, 291–313 (2017). https://doi.org/10.1007/s00360-016-1034-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-016-1034-9