Abstract
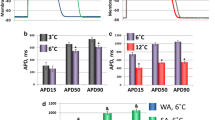
Freshwater fishes of north-temperate latitudes adjust electrical excitability of the heart to seasonal temperature changes by changing expression levels of ion channel isoforms. However, little is known about thermal responses of action potential (AP) in the hearts of marine polar fishes. To this end, we examined cardiac AP in the atrial myocardium of the Arctic navaga cod (Eleginus navaga) from the White Sea (Russia) acclimatized to winter (March) and summer (September) seasons. Acute increases in temperature from 4 to 10 °C were associated with increases in heart rate, maximum velocity of AP upstroke and negative resting membrane potential, while duration of AP was shortened in both winter-acclimatized and summer-acclimatized navaga hearts. In winter, there was a compensatory shortening (41.1 %) of atrial AP duration and this was associated with a strong increase in transcript expression of Erg K+ channels, known to produce the rapid component of the delayed rectifier K+ current, I Kr. Smaller increases were found in the expression of Kir2.1 channels that produce the inward rectifier K+ current, I K1. These findings indicate that the heart of navaga cod has a good acclimatory capacity in electrical excitation of cardiac myocytes, which enables cardiac function in the cold-eurythermal waters of the subarctic White Sea.




Similar content being viewed by others
References
Aho E, Vornanen M (1998) Ca-ATPase activity and Ca-uptake by sarcoplasmic reticulum in fish heart: effects of thermal acclimation. J Exp Biol 201:525–532
Axelsson M (2005) The circulatory system and its control. In: Farrell AP, Steffensen JF (eds) The physiology of polar fishes. Elsevier, San Diego, pp 239–280
Bilyk KT, DeVries AL (2011) Heat tolerance and its plasticity in Antarctic fishes. Comp Biochem Physiol 158A:382–390
Bowler K, Tirri R (1990) Temperature dependence of the heart isolated from the cold or warm acclimated perch (Perca fluviatilis). Comp Biochem Physiol 96A:177–180
DeVries AL, Cheng C-HC (2005) Antifreeze proteins and organismal freezing avoidance in polar fishes. In: Farrell AP, Steffensen JF (eds) The physiology of polar fishes. Elsevier, San Diego, pp 155–201
DeVries AL, Steffensen JF (2005) The Arctic and Antarctic polar marine environments. In: Farrell AP, Steffensen JF (eds) The physiology of polar fishes. Elsevier, San Diego, pp 1–24
Driedzic WR, Gesser H (1994) Energy metabolism and contractility in ectothermic vertebrate hearts: hypoxia, acidosis, and low temperature. Physiol Rev 74:221–258
Eliason EJ, Clark TD, Hague MJ, Hanson LM, Gallagher ZS, Jeffries KM, Gale MK, Patterson DA, Hinch SG, Farrell AP (2011) Differences in thermal tolerance among sockeye salmon populations. Science 332:109–112
Franklin CE, Davison W, Seebacher F (2007) Antarctic fish can compensate for rising temperatures: thermal acclimation of cardiac performance in Pagothenia borchgrevinki. J Exp Biol 210:3068–3074
Gillis TE, Tibbits GF (2002) Beating the cold: the functional evolution of troponin C in teleost fish. Comp Biochem Physiol 134A:763–772
Hassinen M, Paajanen V, Haverinen J, Eronen H, Vornanen M (2007) Cloning and expression of cardiac Kir2.1 and Kir2.2 channels in thermally acclimated rainbow trout. Am J Physiol 292:R2328–R2339
Hassinen M, Haverinen J, Vornanen M (2008a) Electrophysiological properties and expression of the delayed rectifier potassium (ERG) channels in the heart of thermally acclimated rainbow trout. Am J Physiol 295:R297–R308
Hassinen M, Paajanen V, Vornanen M (2008b) A novel inwardly rectifying K+ channel, Kir2.5, is upregulated under chronic cold stress in fish cardiac myocytes. J Exp Biol 211:2162–2171
Hassinen M, Laulaja S, Paajanen V, Haverinen J, Vornanen M (2011) Thermal adaptation of the crucian carp (Carassius carassius) cardiac delayed rectifier current, I Ks, by homomeric assembly of Kv7.1 subunits without MinK. Am J Physiol 301:R255–R265
Haverinen J, Vornanen M (2004) Temperature acclimation modifies Na+ current in fish cardiac myocytes. J Exp Biol 207:2823–2833
Haverinen J, Vornanen M (2007) Temperature acclimation modifies sinoatrial pacemaker mechanism of the rainbow trout heart. Am J Physiol 292:R1023–R1032
Haverinen J, Vornanen M (2009) Responses of action potential and K+ currents to chronic thermal stress in fish hearts. Phylogeny or thermal preferences? Physiol Biochem Zool 82:468–482
Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y (2010) Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev 90:291–366
Hochachka PW, Somero GN (2002) Biochemical adaptation. Oxford University Press, New York
Höglund L, Gesser H (1987) Electrical and mechanical activity in heart tissue of flounder and rainbow trout during acidosis. Comp Biochem Physiol 87A:543–546
Johnson JA, Kelsch SW (1998) Effect of evolutionary thermal environment on temperature-preference relationship in fishes. Env Biol Fishes 53:447–458
Johnston IA, Dunn J (1987) Temperature acclimation and metabolism in ectotherms with particular reference to teleost fish. Symp Soc Exp Biol 41:67–93
Keen JE, Vianzon DM, Farrell AP, Tibbits GF (1994) Effect of temperature and temperature acclimation on the ryanodine sensitivity of the trout myocardium. J Comp Physiol B 164:438–443
Korajoki H, Vornanen M (2013) Temperature-dependence sarco(endo)plasmic reticulum Ca2+-ATPase expression in fish hearts. J Comp Physiol B 183:467–476
Lillywhite HB, Zippel KC, Farrell AP (1999) Resting and maximal heart rates in ectothermic vertebrates. Comp Biochem Physiol 124A:369–382
Lukyanov AN, Sukhova GS, Chudakov LI (1986) Mechanism of variation in the general rhythm of the heart pacemaker in the cod Gadus morhua in response to vagal stimulation. J Evol Biochem Physiol 22:25–30
Miake J, Marban E, Nuss HB (2003) Functional role of inward rectifier current in heart probed by Kir2.1 overexpression and dominant-negative suppression. J Clin Invest 111:1529–1536
Peck L, Morley S, Clark M (2010) Poor acclimation capacities in Antarctic marine ectotherms. Mar Biol 157:2051–2059
Podrabsky JE, Somero GN (2006) Inducible heat tolerance in Antarctic notothenioid fishes. Polar Biol 30:39–43
Pörtner HO (2001) Climate change and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften 88:137–146
Precht H, Christophersen J, Hensel H (1955) Temperatur und Leben. Springer, Berlin
Rosati B, McKinnon D (2009) Structural and regulatory evolution of cellular electrophysiological systems. Evol Dev 11:610–618
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Shiels HA, Di Maio A, Thompson S, Block BA (2011) Warm fish with cold hearts: thermal plasticity of excitation–contraction coupling in bluefin tuna. Proc R Soc Lond B Biol Sci 278:18–27
Sidell BD (2000) Life at body temperatures below 0 °C: the physiology and biochemistry of Antarctic fishes. Gravit Space Biol Bull 13:25–33
Somero GN, DeVries AL (1967) Temperature tolerance of some Antarctic fishes. Science 156:257–258
Stillman J, Somero G (1996) Adaptation to temperature stress and aerial exposure in congeneric species of intertidal porcelain crabs (genus Petrolisthes): correlation of physiology, biochemistry and morphology with vertical distribution. J Exp Biol 199:1845–1855
Vornanen M (1998) L-type Ca current in fish cardiac myocytes: effects of thermal acclimation and β-adrenergic stimulation. J Exp Biol 201:533–547
Vornanen M, Ryökkynen A, Nurmi A (2002a) Temperature-dependent expression of sarcolemmal K+ currents in rainbow trout atrial and ventricular myocytes. Am J Physiol 282:R1191–R1199
Vornanen M, Shiels HA, Farrell AP (2002b) Plasticity of excitation–contraction coupling in fish cardiac myocytes. Comp Biochem Physiol 132A:827–846
Vornanen M, Hassinen M, Koskinen H, Krasnov A (2005) Steady-state effects of temperature acclimation on the transcriptome of the rainbow trout heart. Am J Physiol 289:R1177–R1184
Acknowledgments
We acknowledge Riitta Pietarinen for her excellent technical assistance. Authors thank the director of the White Sea Biological Station Dr. Alexander B. Tzetlin for general support of this study. Authors are also grateful to Valo V. Sivonen and Valentina P. Sivonen for capturing the fish. The study was supported by the Russian Foundation for Basic Research [12-04-31737 to D.V.A.] and a grant from the Academy of Finland (project no. 14795) to MV.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H.V. Carey.
Rights and permissions
About this article
Cite this article
Hassinen, M., Abramochkin, D.V. & Vornanen, M. Seasonal acclimatization of the cardiac action potential in the Arctic navaga cod (Eleginus navaga, Gadidae). J Comp Physiol B 184, 319–327 (2014). https://doi.org/10.1007/s00360-013-0797-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-013-0797-5