Abstract
Objective
To assess the usefulness of combined diffusion kurtosis imaging (DKI) and dynamic contrast-enhanced MRI (DCE-MRI) in the differentiation of parotid gland tumors.
Methods
Seventy patients with 80 parotid gland tumors who underwent DKI and DCE-MRI were retrospectively enrolled and divided into four groups: pleomorphic adenomas (PAs), Warthin tumors (WTs), other benign tumors (OBTs), and malignant tumors (MTs). DCE-MRI and DKI quantitative parameters were measured. The Kruskal–Wallis H test and post hoc test with Bonferroni correction and ROC curve were used for statistical analysis.
Results
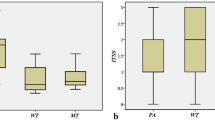
WTs demonstrated the highest Kep value (median 1.89, interquartile range [1.46–2.31] min−1) but lowest Ve value (0.20, [0.15–0.25]) compared with PAs (Kep, 0.34 [0.21–0.55] min−1; Ve, 0.36 [0.24–0.43]), OBTs (Kep, 1.22 [0.27–1.67] min−1; Ve, 0.28 [0.25–0.41]), and MTs (Kep, 0.71 [0.50–1.23] min−1; Ve, 0.35 [0.26–0.45]) (all p < .05). MTs had the lower D value (1.10, [0.88–1.29] × 10−3 mm2/s) compared with PAs (1.81, [1.60–2.20] × 10−3 mm2/s) and OBTs (1.57, [1.32–1.89] × 10−3 mm2/s) (both p < .05). PAs had the lower Ktrans value (0.12, [0.07–0.18] min−1) compared with OBTs (0.28, [0.11–0.50] min−1) (p < .05). The cutoff values of combined Kep and Ve, D, and Ktrans to distinguish WTs, MTs, and PAs sequentially were 1.06 min−1, 0.28, 1.46 × 10−3 mm2/s, and 0.21 min−1, respectively (accuracy, 89% [71/80], 91% [73/80], 78% [62/80], respectively).
Conclusion
The combined use of DKI and DCE-MRI may help differentiate parotid gland tumors.
Key Points
• The combined use of DKI and DCE-MRI could facilitate the understanding of the pathophysiological characteristics of parotid gland tumors.
• A stepwise diagnostic diagram based on the combined use of DCE-MRI parameters and the diffusion coefficient is helpful for accurate preoperative diagnosis in parotid gland tumors and may further facilitate the clinical management of patients.
Similar content being viewed by others
Introduction
Parotid gland tumors (PGTs) contain abundant histological types and subtypes including pleomorphic adenomas (PAs), Warthin tumors (WTs), other benign tumors (OBTs), and malignant tumors (MTs). The treatment strategy and long-term prognosis vary widely depending on the histological type of the tumors [1]. Compared with benign tumors, total parotidectomy with radiotherapy is preferred in the malignancies [2, 3]. In the treatment decision of benign tumors, for patients with PAs, they may require complete surgical excision due to the potential for recurrence and malignant transformation [4], whereas patients with WTs and OBTs, local or superficial parotidectomy, or conservative observation may be sufficient [3]. Therefore, precise preoperative diagnosis is of great importance.
Conventional MRI can delineate lesions concerning the internal structure, morphology, accurate localization, locoregional extension, invasion, and perineural spread of tumors, but the diagnostic performance is limited [3], particularly when benign tumors have similar imaging findings to low-grade malignant tumors showing low signal on T1WI and high signal on T2WI [5]. Advanced MRI techniques like diffusion MRI and perfusion MRI may provide the information of intratumoral water molecular diffusion, microstructural complexity, and capillary blood flows [6, 7]. DKI, a sophisticated modality that quantifies the non-Gaussian behavior of water molecule diffusion, can provide information about heterogeneity and cellularity in vivo with two parameters [8], including diffusion kurtosis (K) and diffusion coefficient (D), and has been proven to be useful in the field of parotid gland lesions [9, 10]. However, DKI used alone may not fully reflect the pathophysiological characteristics of PGTs. Moreover, DCE-MRI monitors T1 changes in tumor tissues over time after contrast administration of gadolinium to quantify the tumor perfusion and vessel permeability [11, 12]. A quantitative evaluation can be made with perfusion parameters including transfer constant (Ktrans), rate constant (Kep), fractional volume of the extravascular extracellular space (Ve), and initial area under the contrast agent concentration–time curve (iAUC) to identify benign and malignant lesions and monitor tumor responses to treatment in the head and neck [7]. Previous studies showed that quantitative DCE-MRI has played an important part in improving the diagnosis of parotid gland lesions [13,14,15]. However, those studies may have some limitations, such as the relatively small sample size. Up to now, none of the studies have demonstrated the added value of combining DKI and DCE-MRI for PGT characterization. We hypothesized that the combined use of DKI and DCE-MRI could reflect the discrepancies in diffusion and perfusion of PGTs. Therefore, the purpose of this study was to evaluate whether DKI and DCE-MRI quantitative parameters are beneficial for differentiating PGTs.
Materials and methods
Patients
The Institutional Review Board of our hospital approved this retrospective study, and the requirement of written informed consent was waived. Between January 2018 and September 2019, a total of 74 consecutive patients with PGTs histologically proven by surgical resection and available DKI and DCE-MRI sequences were retrospectively recruited. Of these patients, 4 were excluded because of MR images with poor quality and obvious motion artifacts (n = 2), and previously treated or recurrent tumors (n = 2). Finally, 70 patients with 80 lesions were included in this study and were divided into four groups including PAs (n = 27), WTs (n = 26), OBTs (n = 17), and MTs (n = 10) (Fig. 1). The OBTs group included 11 basal cell adenoma (BCA), 3 schwannoma, 1 hemolymphangioma, 1 oncocytic adenoma, and 1 cystadenoma. The MTs group included 4 mucoepidermoid carcinoma, 2 lymphoma, 1 carcinoma ex pleomorphic adenoma, 1 acinic cell carcinoma, 1 salivary duct carcinoma, and 1 mammary analog secretory carcinoma.
MRI protocol
All patients underwent MRI on a 3.0-T scanner (Skyra; Siemens Healthcare) with a 20-channel head/neck coil. Conventional MRI protocols were obtained first, then DKI using single-shot echo-planar imaging with fat suppression was performed with b-factors of 0, 1000, 1500, 2000, and 2500 s/mm2 with diffusion gradient applied along all three orthogonal gradient diffusion directions. T1 mapping was performed prior to DCE-MRI sequence. A dose of 0.1 mmol/kg of gadobenate dimeglumine (Gd-DTPA, MultiHance, Bracco Diagnostics) was intravenously administered through the median cubital vein at the rate of 2 ml/s, followed by a 15-ml flush of saline. The parameters of MR sequences are listed in Supplementary Material 1.
Image processing and analysis
The DCE-MRI processing was dealt with the commercial software tool (Tissue 4D, Syngo.via; Siemens Healthcare). The post-processing procedures are demonstrated in Supplementary Material 2.
DKI images were processed using prototype software (Body diffusion toolbox, Siemens Healthcare) and Diffusional Kurtosis Estimator (DKE, version 2.5.1, www.musc.edu/cbi). The DKI model is described as the following formula [16]:
where D represents the diffusion coefficient for non-Gaussian distribution and K represents the diffusion kurtosis coefficient [17].
For evaluation of DCE-MRI data, the values of quantitative parameters were automatically calculated by placing a single region of interest (ROI) manually in the largest enhanced solid portion of the lesion, excluding blood vessel, hemorrhagic, necrotic, and cystic regions referred to T2WI and contrast-enhanced T1WI. The corresponding ROIs were drawn on DKI maps for K and D values using the ImageJ software (version 1.8, National Institutes of Health). Image analysis was conducted separately by two radiologists blinded to histological diagnosis (Y.C. and N.H., with 2 and 8 years of experience in head and neck imaging, respectively). The quantitative measurement results of two readers were used to evaluate the interobserver agreement. The quantitative parameter results measured repeatedly by reader 1 with at least 1-month interval were used to evaluate intraobserver agreement. Finally, the measurement results of reader 1 were used for statistical analysis.
Statistical analysis
All numeric data were tested for normality using Kolmogorov–Smirnov’s test and for variance homogeneity using Levene’s test. The normally distributed variables were expressed as the means ± standard deviation and the non-normally distributed variables were expressed as medians (interquartile ranges, IQRs). A chi-square test was used to compare the discrepancy in sex between benign and malignant tumors. An independent samples t-test was made to compare the difference in age between the two groups. Mann–Whitney U test was used to compare DCE-MRI and DKI quantitative parameters between benign and malignant PGTs. The Kruskal–Wallis H test was made to test overall differences of quantitative parameters among four groups of PGTs. The post hoc test with Bonferroni correction was used for pairwise comparisons when the overall test was statistically significant. The intraclass correlation coefficient (ICC) with 95% confidence intervals (CIs) was used to assess the inter- and intraobserver agreement for quantitative parameters. The ICC > 0.61 was considered good agreement. The receiver operating characteristic (ROC) curve was drawn to ascertain diagnostic performance and optimal cutoff values of quantitative parameters. Then the area under the curve (AUC), sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were further calculated. The combination of Kep and Ve values for differentiating WTs from other three entities was based on the logistic regression analysis. The method developed by DeLong et al. [18] was used for comparisons of AUCs. Statistical analysis was performed using MedCalc statistical software (version 15.8, MedCalc), SPSS software (version 26.0, SPSS), and Graphpad Prism (version 6.0, GraphPad Software). p < 0.05 was considered statistically significant.
Results
Patients characteristics
The main demographic information of patients with PGTs is summarized in Table 1. The statistical results demonstrated that all numeric data were not normally distributed and equal variance except for the age. There was no significant difference in sex (p > 0.99) and age distribution (p = 0.20) between the benign and malignant groups. Fifty-six patients presented with palpable mass without tenderness, 11 patients felt swelling with pain, and the tumor was found during physical examination in 3 patients. One had facial nerve palsy.
DKI and DCE-MRI analysis between benign and malignant PGTs
The comparisons of quantitative parameters between benign and malignant tumors are summarized in Supplementary Material 3 (Table E2). The D value of benign tumors (median 1.50, IQR [1.04–1.86] × 10−3 mm2/s) was significantly higher than that of malignant tumors (1.10, [0.88–1.29] × 10−3 mm2/s) (p = 0.02). The cutoff value of D was 1.24 × 10−3 mm2/s (AUC, 0.73; accuracy, 69% [55/80]) (Supplementary Material 3, Table E3). Additionally, there were insignificant differences in K, Ktrans, Kep, Ve, and iAUC values between the two groups (all p > 0.05).
DKI and DCE-MRI analysis between four groups of PGTs
The Kruskal–Wallis H test revealed that there were statistically significant differences in all quantitative parameters among different groups of PGTs (all p < 0.001), and the comparisons of quantitative parameters of PGTs are summarized in Table 2. For DCE-MRI parameters, as exhibited in Figs. 2, 3, 4, and 5, the Ktrans value of PAs (0.12, [0.07–0.18] min−1) was significantly lower than that of WTs (0.38, [0.28–0.45] min−1), OBTs (0.28, [0.11–0.50] min−1), and MTs (0.30, [0.14–0.50] min−1) (adjusted p < 0.001, = 0.008, and 0.009, respectively). Moreover, the Kep value of WTs (1.89, [1.46–2.31] min−1) was significantly higher than that of PAs (0.34, [0.21–0.55] min−1), OBTs (1.22, [0.27–1.67] min−1), and MTs (0.71, [0.50–1.23] min−1) (adjusted p < 0.001, = 0.01, and 0.047, respectively). Meanwhile, the Ve value of WTs (0.20, [0.15–0.25]) was significantly lower than that of PAs (0.36, [0.24–0.43]), OBTs (0.28, [0.25–0.41]), and MTs (0.35, [0.26–0.45]) (adjusted p < 0.001, = 0.001, and 0.001, respectively). Additionally, the iAUC value of PAs (0.13, [0.08–0.22] mmol·s/kg) was significantly lower than that of WTs (0.25, [0.21–0.30] mmol·s/kg) and MTs (0.32, [0.18–0.37] mmol·s/kg) (adjusted p = 0.002 and 0.007, respectively).
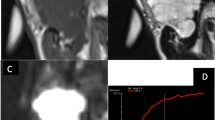
A 67-year-old man with Warthin tumor in the left parotid gland (white arrow). DCE-MRI parameter maps showed the Kep (a), Ve (b), and Ktrans (c) values of 2.02 min−1, 0.19, and 0.38 min−1, respectively. d D map demonstrated that the mass showed hypointensity compared with neighboring normal-appearing muscle, with a D value of 0.90 × 10−3 mm2 /s
A 24-year-old man with mucoepidermoid carcinoma in the right parotid gland (white arrow). DCE-MRI parameter maps showed the Kep (a), Ve (b), and Ktrans (c) values of 0.90 min−1, 0.61, and 0.55 min−1, respectively. d D map demonstrated that the solid portion of the mass showed hypointensity compared with neighboring normal-appearing muscle, with a D value of 1.46 × 10−3 mm2 /s
A 34-year-old man with pleomorphic adenoma in the left parotid gland (white arrow). Dynamic contrast-enhanced MRI parameter maps showed the Kep (a), Ve (b), and Ktrans (c) values of 0.30 min−1, 0.51, and 0.15 min−1, respectively. d D map demonstrated that the mass showed slightly hypointensity compared with neighboring normal-appearing muscle, with a D value of 1.85 × 10−3 mm2 /s
For DKI parameters, the D values of both MTs (1.10, [0.88–1.29] × 10−3 mm2/s) and WTs (0.97, [0.89–1.27] × 10−3 mm2/s) were significantly lower than those of PAs (1.81, [1.60–2.20] × 10−3 mm2/s) (both adjusted p < 0.001 for MTs and WTs) and OBTs (1.57, [1.32–1.89] × 10−3 mm2/s) (adjusted p = 0.03 for MTs and < 0.001 for WTs), respectively. Moreover, the K value of PAs (0.51, [0.47–0.62]) was significantly lower than that of MTs (0.87, [0.69–1.01]) (adjusted p = 0.001); the K value of WTs (0.99, [0.84–1.09]) was higher than that of PAs and OBTs (0.64, [0.56–0.71]) (both adjusted p < 0.001).
The diagnostic performances between the four groups are summarized in Table 3. ROC analyses showed that in differentiating PAs from WTs, K (cutoff value, ≤ 0.72; AUC, 0.99; accuracy, 98% [52/53]) showed optimal diagnostic performance, which was better than that of Ve and iAUC (both p = 0.003), but the difference in AUC between K and Ktrans, Kep, or D did not reach significance (p = 0.08, 0.50, and 0.20, respectively). In differentiating PAs from OBTs, the cutoff value of Ktrans was 0.21 min−1 (AUC, 0.77; accuracy, 80% [35/44]). In differentiating PAs from MTs, D (cutoff value, > 1.46 × 10−3 mm2/s; AUC, 0.96; accuracy, 92% [34/37]) showed optimal diagnostic performance, but the difference in AUC between D and Ktrans, iAUC, or K did not reach significance (p = 0.14, 0.08, and 0.40, respectively). In differentiating WTs from OBTs, K (cutoff value, > 0.72; AUC, 0.97; accuracy, 93% [40/43]) showed optimal diagnostic performance, which was significantly better than that of Kep (p = 0.01), but the difference in AUC between K and Ve or D did not reach significance (p = 0.13 and 0.11, respectively). In differentiating WTs from MTs, Ve (cutoff value, > 0.23; AUC, 0.92; accuracy, 78% [28/36]) showed a higher AUC than Kep, but the difference did not reach significance (p = 0.33). The cutoff value of D was 1.24 × 10−3 mm2/s in differentiating OBTs from MTs (AUC, 0.88; accuracy, 82% [22/27]).
Excellent inter- and intraobserver agreement for DKI and DCE-MRI parameters was achieved with ICCs ranging from 0.92 to 0.99 (see Supplementary Material 4 for details).
DKI and DCE-MRI analysis between solid and predominantly cystic of WTs
To overcome the possible influence in DKI and DCE-MRI parameters caused by heterogeneity of Warthin tumors, we further compared these parameters derived from the solid portion between Warthin tumors with solid and predominantly cystic forms. Of 26 Warthin tumors, 20 (77%) were presented as predominantly solid masses, which included completely solid or solid tumor with small cystic components, and 6 (23%) were presented as predominantly cystic entities. No significant differences were found in all parameters between predominantly solid and predominantly cystic Warthin tumors (p = 0.12, 0.05, 0.70, 0.98, 0.66, and 0.22 for Ktrans, Kep, Ve, iAUC, D, and K value, respectively).
Stepwise discrimination among four groups of PGTs using DKI and DCE-MRI
Initially, PAs, OBTs, and MTs were classified as one group for the reason that there were significant differences in Kep and Ve values between these three groups of tumors and WTs. Both Kep and Ve values helped discriminate WTs from PAs, OBTs, and MTs, with a cutoff value of > 1.06 min−1 for Kep (accuracy, 84% [67/80]) and ≤ 0.28 for Ve (accuracy, 74% [59/80]). Moreover, the combination of Kep and Ve values generated the better diagnostic performance (accuracy, 89% [71/80]) than Ve (AUC, 0.93 vs 0.86; p = 0.03) and was finally applied to distinguish WTs from the other three groups of PGTs (Table 4).
Subsequently, PAs and OBTs were classified as one group for the reason that lower D value was found in MTs than the other two entities. The D value was useful for differentiating MTs from PAs and OBTs, with a cutoff value of ≤ 1.46 × 10−3 mm2/s (accuracy, 85% [46/54]) (Table 4).
Eventually, the Ktrans value was used to differentiating PAs from OBTs resulted from the lower Ktrans value of PAs with a cut-off value of ≤ 0.21 min−1 (accuracy, 80% [35/44]) (Table 4). From this aspect, a stepwise distinguishable diagnostic diagram was put up for discriminating the four groups of PGTs consisting of PAs, WTs, OBTs, and MTs (Table 5 and Fig. 6). The diagnostic diagram provided high accuracy for the differential diagnosis of WTs and MTs of 89% (71/80) and 91% (73/80), respectively, and modest accuracy for both PAs and OBTs of 78% (62/80).
Graph shows stepwise discrimination of four groups of parotid gland tumors, including Warthin tumors, malignant tumors, pleomorphic adenomas, and other benign tumors using DCE-MRI parameters and diffusion coefficient. PA pleomorphic adenoma, WT Warthin tumor, OBT other benign tumor, MT malignant tumor. Units for quantitative parameters: Kep and Ktrans are expressed in min−1; D are expressed in × 10−3 mm2 /s
Discussion
DKI and DCE-MRI have been widely used in head and neck regions [19, 20]. However, to our knowledge, the diagnostic performance of combined DKI and DCE-MRI models in the field of PGTs has not been assessed in the existing studies. Our current study showed that DKI and DCE-MRI can elucidate the diffusion and perfusion characteristics of PGTs and provided a stepwise diagnostic diagram to distinguish common PGTs with modest to high accuracy (78–91%). These findings suggest that DKI and DCE-MRI quantitative parameters may facilitate the understanding of the pathophysiological characteristics of PGTs.
The D value derived from DKI is the corrected diffusion-related coefficient for non-Gaussian bias, and there is a strong inverse association with the tumor cellular density and nucleus-to-cytoplasm ratios [19]. Previously, Qian et al. [10] showed that no significant difference was found in the D value between the benign and the malignant tumors. They implied that low D value in WTs mainly accounted for low D value in benign PGTs and there was possibly a slight difference of D value by increasing the sample size. In this study, with a relatively big sample size, the mean D value was the only significant parameter in distinguishing the benignity from the malignancy, which was consistent with the hypothesis. In subgroup comparisons, a previous study [9] demonstrated that PAs had higher D value whereas WTs and MTs had relatively lower D value, which was in good agreement with our results. Histologically, the high D value in PAs resulted from the abundant myxoid and chondroid matrices [10]. Flourishing cells, enlarged nuclei, and smaller extracellular space in MTs [21] and high cellularity that resulted from rich lymphoid tissue–related interstitium in WTs [22, 23] help explain the low D value. Notably, in this study, D was used for the second step in the stepwise diagnostic diagram instead of K, due to the fact that the difference of K value between OBTs and MTs was not statistically significant, which was incompatible with the previous study [9]. The chief reasons for it may be different histopathological subtypes of OBTs and MTs and different b-value selection in DKI scan parameters in the two studies [24]. However, ROC curve analysis showed that the K value can provide a high accuracy in differentiating WTs from PAs and OBTs. The K value can quantify the degree of non-Gaussian distribution and tend to move together with cellular heterogeneity and tissue complexity [21]. The higher K value of WTs might be attributed to the complex microstructure within tumor, including various proportions of epithelia with papillary proliferation, lymphoid tissue, and cystic components filled with mucoid in histopathology [25]. Hence, our findings suggest that the K value evaluated preoperatively may aid in differentiating PGTs.
Ktrans derived from DCE-MRI chiefly depends on blood flow in tissue and capillary permeability [19, 26]. Our findings discovered that Ktrans was the only useful imaging marker for distinguishing PAs from OBTs, which was inconsistent with the previous study [14]. In our study, BCAs accounted for the majority of OBTs, which possibly to some extent affected the Ktrans value in the OBTs. Hence, the significant differences in Ktrans value were related to the comparative higher Ktrans value of BCAs. Histologically, compared with PAs, BCAs are short of mesenchymal component and chondromyxoid stroma but have a great deal of vascular architecture [27]. The different histological features may be the dominant reason; another one may be various pathological types but small sample size in OBTs in our study. Further studies with a bigger sample size are mandatory to analyze the differences of quantitative parameters between PAs and OBTs. Ve has a strong correlation with tissue necrosis and cellularity and Kep is equal to the ratio of Ktrans to Ve [19, 26]. In our study, WTs had the highest Kep value but lowest Ve value than the other three groups, which was in accord with the previous study [13, 14]. In the study by Yabuuchi et al. [13], the differences in Kep and Ve values reached statistical significance among four types of PGTs and particularly, the use of the Kep value extremely promoted the diagnostic efficacy in the decision tree analysis. Histologically, limited extracellular and extravascular space in WTs [14] results in high Kep value but low Ve value. Accordingly, with the characteristic presentations of perfusion parameters (high Kep value and low Ve value), WTs could be effectively differentiated from the other three groups.
In our current study, the improved diagnostic performance was found in the combination of Kep and Ve values for differentiating WTs from the other three groups, indicating Kep and Ve values could be the optimal quantitative parameters for the diagnosis of WTs. Moreover, favorable sensitivity, specificity, and accuracy were also investigated in D value for the differentiation of MTs from PAs and OBTs, implying D value may conduce to the differential diagnosis. Furthermore, our study showed that Ktrans value avails to further discriminating PAs from OBTs. As a result, in the manner of the stepwise diagram combining DCE-MRI- and DKI-derived parameters, these common histological types of PGTs could be well differentiated. Regardless, the validation and correction of the criteria still need to be made with a larger sample size in further studies.
There are several limitations to our study. First, this was a retrospective study that may generate bias in case selection and the sample size of patients with malignant tumor was relatively small. Further study with a larger sample size is required to confirm our results. Second, magnetic susceptibility artifacts with anatomic distortions may inevitably exert an influence on the measurements and corresponding results. Various available DKI sequences like the readout-segmented echo-planar imaging (RS-EPI) technique may be offered as a solution. Third, our study applied DKI and DCE-MRI for PGTs. Further studies with multimodal imaging using intravoxel incoherent motion (IVIM) and arterial spin labeling (ASL) will improve the results.
In conclusion, our study demonstrated that DKI and DCE-MRI are useful for characterizing common PGTs. Additionally, a stepwise diagnostic diagram was put up based on the combined use of DCE-MRI parameters and the diffusion coefficient to improve the diagnostic ability. In the future, studies with a more abundant and larger sample size are necessary to help scrutinize, optimize, and validate our stepwise diagram and expand the applications of DKI and DCE-MRI to other parotid gland diseases.
Abbreviations
- BCA:
-
Basal cell adenoma
- D :
-
Diffusion coefficient
- DCE-MRI:
-
Dynamic contrast-enhanced MRI
- DKI:
-
Diffusion kurtosis imaging
- iAUC:
-
Initial area under the contrast agent concentration–time curve
- IQR:
-
Interquartile range
- K :
-
Diffusion kurtosis
- K ep :
-
Rate constant from extravascular extracellular space to plasma
- K trans :
-
Transfer constant from plasma to extravascular extracellular space
- MT:
-
Malignant tumor
- OBT:
-
Other benign tumor
- PA:
-
Pleomorphic adenoma
- PGT:
-
Parotid gland tumor
- V e :
-
Fractional volume of the extravascular extracellular space
- WT:
-
Warthin tumor
References
Gatta G, Guzzo M, Locati L, McGurk M, Prott F (2020) Major and minor salivary gland tumours. Crit Rev Oncol Hematol 152:102959
Khalek Abdel Razek A (2018) Characterization of salivary gland tumours with diffusion tensor imaging. Dentomaxillofac Radiol 47:20170343
Gökçe E (2020) Multiparametric magnetic resonance imaging for the diagnosis and differential diagnosis of parotid gland tumors. J Magn Reson Imaging 52:11–32
Hellquist H, Paiva-Correia A, Vander Poorten V et al (2019) Analysis of the clinical relevance of histological classification of benign epithelial salivary gland tumours. Adv Ther 36:1950–1974
Som P, Biller H (1989) High-grade malignancies of the parotid gland: identification with MR imaging. Radiology 173:823–826
Meng N, Wang X, Sun J et al (2021) A comparative study of the value of amide proton transfer-weighted imaging and diffusion kurtosis imaging in the diagnosis and evaluation of breast cancer. Eur Radiol 31:1707–1717
Jiang J, Xiao Z, Tang Z, Zhong Y, Qiang J (2018) Differentiating between benign and malignant sinonasal lesions using dynamic contrast-enhanced MRI and intravoxel incoherent motion. Eur J Radiol 98:7–13
Zheng D, Lai G, Chen Y et al (2018) Integrating dynamic contrast-enhanced magnetic resonance imaging and diffusion kurtosis imaging for neoadjuvant chemotherapy assessment of nasopharyngeal carcinoma. J Magn Reson Imaging 48:1208–1216
Yu S, Zhang Z, Bao Q et al (2018) Diffusion kurtosis imaging in the differential diagnosis of parotid gland disease and parotid adenolymphoma: preliminary results. Dentomaxillofac Radiol 47:20170388
Qian W, Xu X, Zhu L et al (2019) Preliminary study of using diffusion kurtosis imaging for characterizing parotid gland tumors. Acta Radiol 60:887–894
Konar A, Vajuvalli N, Rao R, Jain D, Ramesh Babu D, Geethanath S (2020) Accelerated dynamic contrast enhanced MRI based on region of interest compressed sensing. Magn Reson Imaging 67:18–23
Zheng N, Li R, Liu W, Shao S, Jiang S (2018) The diagnostic value of combining conventional, diffusion-weighted imaging and dynamic contrast-enhanced MRI for salivary gland tumors. Br J Radiol 91:20170707
Yabuuchi H, Kamitani T, Sagiyama K et al (2020) Characterization of parotid gland tumors: added value of permeability MR imaging to DWI and DCE-MRI. Eur Radiol 30:6402–6412
Xu Z, Zheng S, Pan A, Cheng X, Gao M (2019) A multiparametric analysis based on DCE-MRI to improve the accuracy of parotid tumor discrimination. Eur J Nucl Med Mol Imaging 46:2228–2234
Patella F, Franceschelli G, Petrillo M et al (2018) A multiparametric analysis combining DCE-MRI- and IVIM -derived parameters to improve differentiation of parotid tumors: a pilot study. Future Oncol 14:2893–2903
Rosenkrantz A, Padhani A, Chenevert T et al (2015) Body diffusion kurtosis imaging: basic principles, applications, and considerations for clinical practice. J Magn Reson Imaging 42:1190–1202
Zhang Q, Peng Y, Liu W et al (2020) Radiomics based on multimodal MRI for the differential diagnosis of benign and malignant breast lesions. J Magn Reson Imaging 52:596–607
DeLong E, DeLong D, Clarke-Pearson D (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Xiao Z, Tang Z, Qiang J et al (2018) Differentiation of olfactory neuroblastomas from nasal squamous cell carcinomas using MR diffusion kurtosis imaging and dynamic contrast-enhanced MRI. J Magn Reson Imaging 47:354–361
Minosse S, Marzi S, Piludu F et al (2020) Diffusion kurtosis imaging in head and neck cancer: a correlation study with dynamic contrast enhanced MRI. Phys Med 73:22–28
Ma G, Xu X, Hu H et al (2018) Utility of readout-segmented echo-planar imaging-based diffusion kurtosis imaging for differentiating malignant from benign masses in head and neck region. Korean J Radiol 19:443–451
Munhoz L, Ramos E, Im D et al (2019) Application of diffusion-weighted magnetic resonance imaging in the diagnosis of salivary gland diseases: a systematic review. Oral Surg Oral Med Oral Pathol Oral Radiol 128:280–310
Yamamoto T, Kimura H, Hayashi K, Imamura Y, Mori M (2018) Pseudo-continuous arterial spin labeling MR images in Warthin tumors and pleomorphic adenomas of the parotid gland: qualitative and quantitative analyses and their correlation with histopathologic and DWI and dynamic contrast enhanced MRI findings. Neuroradiology 60:803–812
Chuhutin A, Hansen B, Jespersen S (2017) Precision and accuracy of diffusion kurtosis estimation and the influence of b-value selection. NMR Biomed. https://doi.org/10.1002/nbm.3777
Ikeda M, Motoori K, Hanazawa T et al (2004) Warthin tumor of the parotid gland: diagnostic value of MR imaging with histopathologic correlation. AJNR Am J Neuroradiol 25:1256–1262
Tofts P, Brix G, Buckley D et al (1999) Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging 10:223–232
Shi L, Wang Y, Yu C, Zhao F, Kuang P, Shao G (2012) CT and ultrasound features of basal cell adenoma of the parotid gland: a report of 22 cases with pathologic correlation. AJNR Am J Neuroradiol 33:434–438
Acknowledgements
The authors thank Xiao et al. and their published article (Zebin X et al. Laryngoscope 130(12): E727-E735) for providing a strong reference of differential and stepwise protocol. The authors thank Zhongshuai Zhang, PhD., at Siemens Healthcare Ltd. (Z.Z.), Shanghai, China, for language polishing.
Funding
This study was supported by the Startup Fund for Scientific Research, Fujian Medical University (No.2019QH1105).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Prof. Dairong Cao, MD, The First Affiliated Hospital of Fujian Medical University.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects and cohort overlap
Some patients in the present study cohort overlapped one of our studies, in which we used readout segmentation of long variable echo-trains diffusion-weighted imaging and quantitative dynamic contrast-enhanced MRI (DCE-MRI) with ROI measurements to differentiate parotid tumors. As for the DCE-MRI part, 69 cases (56.1%) overlapped in these two studies. In that study, the temporal resolution, duration of dynamic sequence and diffusion technique, and calculated mode were far different from our present study.
Methodology
• Retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nan Huang and Yu Chen are co-first authors and contributed equally to this study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, N., Chen, Y., She, D. et al. Diffusion kurtosis imaging and dynamic contrast-enhanced MRI for the differentiation of parotid gland tumors. Eur Radiol 32, 2748–2759 (2022). https://doi.org/10.1007/s00330-021-08312-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08312-y