Abstract
Objectives
In recognition of the significant impairment caused by haemoptysis on a patient’s quality of life, bronchial artery embolisation has been introduced worldwide as one of the first-line treatment options. Since little evidence is available on the mechanisms of recurrent haemoptysis after super-selective bronchial artery coil embolisation (ssBACE), the purpose of the present study is to evaluate these.
Methods
We retrospectively evaluated the mechanisms of recurrent haemoptysis using both enhanced computed tomography and cineangiography following ssBACE by reviewing 299 haemoptysis-related arteries (HRAs) in 57 consecutive patients who underwent 2nd series ssBACE for the management of recurrent haemoptysis between April 2010 and December 2015.
Results
Median age of patients was 69 (interquartile range 64–74) years, and 43.9% were men. This study revealed that (1) recanalisation was the most common mechanism (45.2%) followed by development of new HRA (38.5%), bridging collaterals (14.7%) and conventional collaterals (1.7%); (2) these trends could be modified in several situations such as with antiplatelet or anticoagulant medications; (3) relatively large-diameter HRAs were more likely to recanalise compared with small-diameter HRAs and (4) recurrent haemoptysis could be managed by 2nd series ssBACE with a procedural success rate of 97.7% without any major complications.
Conclusions
Recanalisation was the most common mechanism of recurrent haemoptysis after ssBACE. Our results provide interventionists with indispensable insights.
Key Points
• Recanalisation was the most common mechanism of recurrent haemoptysis after super-selective bronchial artery coil embolisation, followed by development of new haemoptysis-related arteries
• These trends could be modified in several situations such as with antiplatelet or anticoagulant medications
• Recurrent haemoptysis could be managed by 2nd series super-selective bronchial artery coil embolisation with a procedural success rate of 97.7% without any major complications.
Similar content being viewed by others
Introduction
Haemoptysis affects patients with a wide variety of respiratory diseases, such as bronchiectasis, non-tuberculous mycobacterium (NTM) pulmonary infection and pulmonary aspergillosis [1,2,3,4,5,6]. As a result of the significant negative impact of massive haemoptysis on a patient’s quality of life and risk of mortality, an interventional radiological approach known as bronchial artery embolisation (BAE) has been introduced and evaluated extensively [7,8,9,10,11,12,13,14]. In addition, interest has recently started to shift to long-term haemoptysis-free survival rather than short-term haemostatic rate, since short-term haemostasis can be achieved in the majority of cases owing to advancements in interventional devices and medical knowledge [9,10,11,12,13,14,15,16,17]. For example, BAE performed using polyvinyl alcohol (PVA) or N-butyl-2-cyanoacrylate (NBCA), or using a metallic coil, has been shown to produce much more favourable long-term outcomes compared to BAE performed using a gelatin sponge [14, 16]. The largest observational studies to date have shown that the estimated 1-year haemoptysis-free survival rates for BAE performed using PVA, NBCA and a metallic coil are 77%, 88% and 87%, respectively [14, 16]. However, little evidence is available on the mechanisms of recurrent haemoptysis after BAE using metallic coil [11,12,13,14,15,16,17]. The aim of the present study was to evaluate the mechanisms of recurrent haemoptysis using both enhanced-computed tomography (e-CT) and cineangiography after super-selective bronchial artery coil embolisation (ssBACE) by reviewing 299 haemoptysis-related arteries (HRAs) from 57 consecutive patients who underwent 2nd series ssBACE for the management of recurrent haemoptysis.
Materials and methods
Study patients and data collection
This single-centre retrospective study included 57 consecutive patients who underwent 2nd series ssBACE for the management of recurrent haemoptysis after 1st series elective ssBACE (n = 489) at our institution between April 2010 and December 2015 [16]. As previously reported, we defined haemoptysis and recurrent haemoptysis as airway bleeding with an estimated volume of greater than 20 cm3. In this study, ssBACE was indicated for patients with severe impairments to daily quality of life regardless of the amount of haemoptysis [16]. We collected data on all the variables shown in tables and figures retrospectively from the patient records. In the present study, underlying diseases included bronchiectasis, NTM pulmonary infection, pulmonary aspergillosis, pulmonary tuberculosis (Tb) sequelae and cryptogenic haemoptysis. Exacerbation of underlying diseases was evaluated by the consensus of two pulmonologists on the basis of CT findings. We defined exacerbation as being present when the number or extent of disease-related findings increased in the lung parenchyma on CT at 2nd series ssBACE compared with the findings at 1st series ssBACE. For example, exacerbation of bronchiectasis was determined if new enlargement or increase of dilated bronchus was present. Decision was withheld if the presence of blood significantly impaired accurate interpretation by masking lung parenchymal changes because of exacerbation of underlying diseases (n = 1). The study protocol complied with the standards outlined in the Declaration of Helsinki and was approved by the institutional ethical committee (approval number 2017-03). The requirement of written informed consent was waived because of the retrospective nature of the study. Several cases have previously been published as image-content sharing case reports in another journal [18].
Standard ssBACE procedure and classification of mechanisms
The standard ssBACE procedure and identification protocol for HRAs have previously been reported elsewhere and are summarised shortly as follows [16]. During 1st series ssBACE, HRAs identified using e-CT were embolised using three detachable or pushable platinum coils (IDC® or Interlock® Boston Scientific Japan, Target® Stryker Japan, Nester® Cook Japan, C-STOPPER® PIOLAX). The first coil deployment was performed for anchoring, followed by filling and finishing coil deployments [16]. Before the 2nd series ssBACE, all patients underwent e-CT angiography again to evaluate possible HRAs for procedural planning [5, 6, 9, 16, 19]. Primary signs of a possible HRA consisted of dilatation of the vessel compared to the normal size for that site, tortuosity of the vessels, direct shunting of vessels, aneurysmal formation, pleural adhesion, ground glass opacity suggesting inhaled blood, and new enhancement of the distal part of the HRA embolised at the 1st series ssBACE [1, 5, 6, 8, 9, 16]. Considering the normal arterial vessel size, we defined relatively large-diameter HRAs as those occurring in the bronchial, intercostal and internal thoracic arteries, and relatively small-diameter HRAs as those occurring in all other arteries. All possible HRAs identified using e-CT were super-selectively evaluated using arteriography with 5-Fr guiding catheter and 3-Fr microcatheter systems, with 0.014- or 0.016-inch guide wires, during the 2nd series ssBACE [16]. Aortography was not performed. Following the comprehensive evaluation of e-CT and cineangiography, we classified the mechanisms of recurrent haemoptysis into four categories (as shown in Fig. 1): (1) recanalisation, (2) conventional collaterals, (3) bridging collaterals and (4) new HRA. This last category included vessels that were overlooked (n = 4) or ones that could not be embolised (n = 3) during the 1st series ssBACE. We defined conventional collaterals as instances where the distal part of an embolised HRA is fed blood from vessels other than the proximal part of the embolised HRA. Conversely, we defined bridging collaterals as instances when the distal part of an embolised HRA is fed blood directly from the proximal part of the embolised HRA. Second series ssBACE was performed at the proximal part of previously embolised HRAs if recanalisation or bridging collaterals were present, whereas for newly developed HRAs or if conventional collaterals were present, 2nd series ssBACE was performed in the same manner as the 1st series ssBACE. Procedural success rate was calculated as the number of successfully embolised HRAs divided by the total number of target HRAs during the ssBACE procedure. The definition of major and minor complications was based on guidelines from the Society of Interventional Radiology Standards of Practice Committee [20].
Schematic diagram of mechanisms of recurrent haemoptysis after ssBACE. White arrows indicate blood flow. HRA is successfully embolised after 1st series ssBACE (a). Four possible mechanisms of recurrent haemoptysis include recanalisation (b), bridging and conventional collaterals (c) and the development of new HRA (d). We defined conventional collaterals as when the distal part of an embolised HRA receives blood from other vessels rather than the proximal part of the embolised HRA. HRA haemoptysis-related artery, ssBACE super-selective bronchial artery coil embolisation
Statistical analysis
Our primary outcome of interest was the frequency of occurrence of each mechanism of recurrent haemoptysis. The incidence of each mechanism was counted on the basis of HRA (n = 299). Continuous variables were summarised using medians and interquartile ranges (quartiles 1 to 3), and categorical variables were summarised by percentages. Differences in the incidence of each mechanism between categories were evaluated using a Fisher’s exact test, and those among underlying diseases or vessel types were evaluated, focusing on whether recanalisation is more common as a cause of recurrent haemoptysis than any other cause. By contrast, differences in continuous variables were evaluated using a Kruskal–Wallis test. All statistical analyses were performed using Microsoft R open software (version 3.3.2).
Results
Patients’ characteristics at the time of the 1st ssBACE are shown in Table 1. The median age of patients was 69 (64–74) years old, and 43.9% were men. The most common underlying disease of recurrent haemoptysis after ssBACE was bronchiectasis (38.6%), followed by NTM pulmonary infection (35.1%) and pulmonary aspergillosis (17.5%). The median time from the 1st ssBACE to recurrent haemoptysis was 218 (57–452) days, and there were significant differences among underlying diseases (p = 0.020): the shortest time was 7 (5–9) days for patients with cryptogenic haemoptysis, and the longest time was 354 (165–534) days for patients with NTM pulmonary infection. The number of HRAs was 5 (3–8) and 50% of patients showed exacerbation of underlying diseases at 2nd series ssBACE. The procedural success rate of 2nd series ssBACE was 97.7%, and no major and one minor complication of slight asymptomatic mediastinal haematoma due to wire perforation of an internal thoracic artery which did not need additional management were reported. Regarding the characteristics of HRAs, relatively large-diameter arteries, such as the bronchial (29.8%), intercostal (28.8%) and internal thoracic (13.0%) arteries, were the most common sites of HRAs during the 2nd series ssBACE.
The mechanisms of recurrent haemoptysis are shown in Fig. 2 and Table 2. In general, recanalisation (45.2%) of HRAs was the most common cause in patients with recurrent haemoptysis, followed by new HRA (38.5%), bridging collaterals (14.7%) and conventional collaterals (1.7%) (Fig. 2A). We have presented representative cases with recanalisation and bridging collaterals in Fig. 3 for reference. In a retrospective review of all new HRAs, four could be identified using e-CT evaluation at the 1st series ssBACE. The new HRA category includes the vessels that were overlooked (n = 4) or ones that could not be embolised (n = 3) at the time of the 1st series ssBACE. Several subsets of patients, such as those with no exacerbation of underlying disease (Fig. 2B) and those receiving an anticoagulant (Fig. 2C) or antiplatelet agent (Fig. 2D), showed statistically significant different trends.
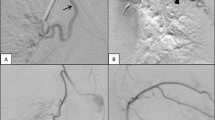
Representative cases of recanalisation and bridging collateral after ssBACE. Representative cineangiography findings of recanalisation (a–d) and bridging collateral (e–h) after 1st series ssBACE. Cineangiography revealed a dilated tortuous left bronchial (a) and right 7th ICA (e) with a bronchopulmonary shunt, which were embolised using metallic coil deployment during 1st series ssBACE (b and f; white arrows). For the treatment of recurrent haemoptysis, the patient underwent 2nd series ssBACE where cineangiography demonstrated a recanalisation (c; yellow arrows) or bridging collateral (g; yellow arrows) of the first embolised part of the HRA. Second series ssBACE was performed successfully at the proximal site of these HRAs (d and h; white arrows). ICA intercostal artery, HRA haemoptysis-related artery, ssBACE super-selective bronchial artery coil embolisation
The fact that relatively large-diameter arteries were the most common sites of HRAs is reflected by the general trend of mechanisms contributing to recurrent haemoptysis. Conversely, the most common mechanism of recurrent haemoptysis in relatively small-diameter arteries, such as the inferior phrenic, supreme intercostal, lateral thoracic, thoracoacromial or other arteries, was the development of new HRAs rather than recanalisation (p < 0.001; Table 2).
Discussion
In the present study, we evaluated the mechanisms of recurrent haemoptysis after ssBACE by reviewing 299 HRAs from 57 patients. The main findings were that (1) recanalisation (45.2%) was the most common mechanism followed by the development of new HRAs (38.5%); (2) these trends differed according to whether patients were on antiplatelet or anticoagulant medications; (3) relatively large-diameter arteries were the most common sites of HRAs, and tended to recanalise more often compared to relatively small-diameter arteries and (4) recurrent haemoptysis could be managed by 2nd series ssBACE with a procedural success rate of 97.7% without any major complications. Since our study is the first to evaluate the mechanisms of recurrent haemoptysis after ssBACE, we believe that our results provide interventionists with indispensable insights for the management of haemoptysis. Further, the findings of this study will significantly improve prognosis and quality of life in patients who suffer from haemoptysis.
Mechanism of recurrent haemoptysis
The most important finding of the present study concerns the mechanisms of recurrent haemoptysis after ssBACE. Recanalisation and the development of new HRAs caused more that 80% of the HRAs at the 2nd series ssBACE. In contrast to PVA and NBCA, which serve as bloodstream-dependent linear liquid embolisation materials, ssBACE provides patients with controlled pinpoint embolisation of target HRAs [10,11,12,13,14,15,16,17]. In light of this, we speculate that the most common mechanism of recurrent haemoptysis, namely recanalisation, may occur more frequently during ssBACE compared to BAE performed with PVA or NBCA. However, the mechanisms of recurrent haemoptysis following BAE using PVA or NBCA have yet to be investigated [10,11,12,13,14,15,16,17]. We also demonstrated that the development of new HRAs was the second most common cause of recurrent haemoptysis in this study, in which 108 (93.9%) were new development of HRA. Even though haemoptysis recurred in an unexpectedly short time period in two patients with cryptogenic haemoptysis (7 [5–9] days; Table 1), this may have been due to a rapid change of bloodstream distribution after the 1st series ssBACE because we could not identify these HRAs in a retrospective review of e-CT or cineangiography at 1st series ssBACE.
Different mechanisms in specific situations
We also revealed that the mechanism of recurrent haemoptysis was significantly different among several subsets of patients. For example, new HRA was the most common mechanism for patients without exacerbation of underlying diseases, as well as in patients receiving an antiplatelet agent. These findings suggest that recurrent haemoptysis could potentially be controlled in certain cases using medication, but the clinical application of this management strategy requires further evaluations. In a subgroup analysis, the most common mechanism was different between large- and small-diameter HRAs. It is understandable that ssBACE of relatively large-diameter vessels could lead to low-density-coil deployment and result in a high incidence of recanalisation. To avoid this kind of recanalisation, or to reduce the incidence of recurrent haemoptysis, we speculate that a high-packing-density-coil deployment manoeuvre, using a hydrogel-polymer-coated platinum coil or a high thrombogenic coil, might be pertinent issues in this field. The efficacy of this manoeuvre has already been shown in neurovascular and gastrointestinal areas, as well as in the treatment of pulmonary arteriovenous malformations [21,22,23].
Clinical implication
Our study has important practical clinical implications. First, one of the most effective strategies towards improving outcomes in patients with haemoptysis would be to reduce the number of recanalisation events after ssBACE, because we have shown that this is the most common mechanism of recurrent haemoptysis. The evaluation of recently introduced hydrogel-polymer-coated platinum coils is anticipated in future studies [21,22,23]. Second, we revealed that 2nd series ssBACE can be performed with a 97.7% procedural success rate even if the proximal part of HRAs was embolised during 1st series ssBACE. In this regard, it is important to remind that 1st series ssBACE should be performed saving some space proximally for additional coil deployment in possible future recanalisation risk. Considering that extreme caution should be exercised when using bloodstream-dependent liquid embolisation materials, owing to the risk of misembolisation particularly in the spinal branches [24, 25], the repeatability of controlled pinpoint embolisation of ssBACE can be a strength for safe and effective BAE procedures in theory [15,16,17].
Study limitations
Our study was retrospective and observational in nature, and it was conducted at a single site. In addition, we analysed data with the assumption that each HRA measurement would be independent because our interest lay in the phenomenon that occurred in each HRA. Thus, statistical significance might have been overestimated compared with patient-based analysis. However, this study examined the largest number of recurrent haemoptysis patients to date and provides the first comprehensive evaluation of the mechanisms of recurrent haemoptysis after ssBACE. These strengths, we feel, outweigh the limitations of the present study.
Conclusions
Recanalisation was the most common mechanism of recurrent haemoptysis after ssBACE. Our results provide interventionists with indispensable insights for the management of haemoptysis.
Abbreviations
- BAE:
-
Bronchial artery embolisation
- e-CT:
-
Enhanced-computed tomography
- HRAs:
-
Haemoptysis-related arteries
- NBCA:
-
N-butyl-2-cyanoacrylate
- NTM:
-
Non-tuberculous mycobacterium
- PVA:
-
Polyvinyl alcohol
- ssBACE:
-
Super-selective bronchial artery coil embolisation
References
Swanson KL, Johnson CM, Prakash UB, McKusick MA, Andrews JC, Stanson AW (2002) Bronchial artery embolization: experience with 54 patients. Chest 121:789–795
Govind M, Maharajh J (2013) The impact of coinfection with human immunodeficiency virus and pulmonary tuberculosis on the success of bronchial artery embolization. Br J Radiol 86:20120256
Lee MK, Kim SH, Yong SJ et al (2015) Moderate hemoptysis: recurrent hemoptysis and mortality according to bronchial artery embolization. Clin Respir J 9:53–64
Poyanli A, Acunas B, Rozanes I et al (2007) Endovascular therapy in the management of moderate and massive haemoptysis. Br J Radiol 80:331–336
Yoon W, Kim JK, Kim YH, Chung TW, Kang HK (2002) Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics 22:1395–1409
Ittrich H, Klose H, Adam G (2015) Radiologic management of haemoptysis: diagnostic and interventional bronchial arterial embolisation. Rofo 187:248–259
Remy J, Arnaud A, Fardou H, Giraud R, Voisin C (1977) Treatment of hemoptysis by embolization of bronchial arteries. Radiology 122:33–37
Vujic I, Pyle R, Hungerford GD, Griffin CN (1982) Angiography and therapeutic blockade in the control of hemoptysis. The importance of nonbronchial systemic arteries. Radiology 143:19–23
Bruzzi JF, Rémy-Jardin M, Delhaye D, Teisseire A, Khalil C, Rémy J (2006) Multi-detector row CT of hemoptysis. Radiographics 26:3–22
Kato A, Kudo S, Matsumoto K et al (2000) Bronchial artery embolization for hemoptysis due to benign diseases: immediate and long-term results. Cardiovasc Intervent Radiol 23:351–357
Chun JY, Belli AM (2010) Immediate and long-term outcomes of bronchial and non-bronchial systemic artery embolisation for the management of haemoptysis. Eur Radiol 20:558–565
Hahn S, Kim YJ, Kwon W, Cha SW, Lee WY (2010) Comparison of the effectiveness of embolic agents for bronchial artery embolization: gelfoam versus polyvinyl alcohol. Korean J Radiol 11:542–546
Yoo DH, Yoon CJ, Kang SG, Burke CT, Lee JH, Lee CT (2011) Bronchial and nonbronchial systemic artery embolization in patients with major hemoptysis: safety and efficacy of N-butyl cyanoacrylate. AJR Am J Roentgenol:W199–W204
Woo S, Yoon CJ, Chung JW et al (2013) Bronchial artery embolization to control hemoptysis: comparison of N-butyl-2-cyanoacrylate and polyvinyl alcohol particles. Radiology 269:594–602
Okuda K, Masuda K, Kawashima M et al (2016) Bronchial artery embolization to control hemoptysis in patients with Mycobacterium avium complex. Respir Investig 54:50–58
Ishikawa H, Hara M, Ryuge M et al (2017) Efficacy and safety of super-selective bronchial artery coil embolization for hemoptysis: a single center retrospective observational study. BMJ Open 7:e014805
Ando T, Kawashima M, Masuda K et al (2017) Clinical and angiographic characteristics of 35 patients with cryptogenic hemoptysis. Chest 152:1008–1014
Ishikawa H (2016) J Typ Med Images Video Case ID 147, 148 and 156. http://thejtmiv.com. Accessed 21 May 2018
Abal AT, Nair PC, Cherian J (2001) Haemoptysis: aetiology, evaluation and outcome–a prospective study in a third-world country. Respir Med 95:548–552
Angle JF, Siddiqi NH, Wallace MJ et al (2010) Quality improvement guidelines for percutaneous transcatheter embolization: Society of Interventional Radiology Standards of Practice Committee. J Vasc Interv Radiol 21:1479–1486
White PM, Lewis SC, Gholkar A, HELPS trial collaborators et al (2011) Hydrogel-coated coils versus bare platinum coils for the endovascular treatment of intracranial aneurysms (HELPS): a randomised controlled trial. Lancet 377:1655–1662
Maleux G, Deroose C, Fieuws S et al (2013) Prospective comparison of hydrogel-coated microcoils versus fibered platinum microcoils in the prophylactic embolization of the gastroduodenal artery before yttrium-90 radioembolization. J Vasc Interv Radiol 24:797–803
Shimohira M, Kawai T, Hashizume T, Muto M, Kitase M, Shibamoto Y (2018) Usefulness of hydrogel-coated coils in embolization of pulmonary arteriovenous malformations. Cardiovasc Intervent Radiol. https://doi.org/10.1007/s00270-018-1876-5
Schrodt JF, Becker GJ, Scott JA, Warren CH, Benenati SV (1987) Bronchial artery embolization: monitoring with somatosensory evoked potentials. Work in progress. Radiology 164:135–139
Brown AC, Ray CE (2012) Anterior spinal cord infarction following bronchial artery embolization. Semin Interv Radiol 29:241–244
Acknowledgements
We express our gratitude to Dr. Shigeo Shirakawa (president of Seiwakai Hospital Group), Mr. Unshow Shirakawa (vice president of Seiwakai Hospital Group), Toru Onishi, Daiki Mukai, Yumiko Yamamoto and the Japan Society of Clinical Research for their dedicated support of our work.
Funding
Academic and educational support for this study was provided by the Japan Society of Clinical Research, which received sponsorship from Terumo, Tokyo, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Hideo Ishikawa.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper, but the corresponding author (Masahiko Hara) has significant statistical expertise.
Informed consent
Written informed consent was waived by the institutional review board.
Ethical approval
Institutional review board approval was obtained.
Study subjects or cohorts overlap
Data of all 57 patients were reported previously [please refer to refs. 16 and 18]. However, previous manuscripts evaluated haemoptysis-free survival after 1st ssBACE or just reported three patients as image-content sharing case reports. Thus, the current analysis is totally different from those previous reports because the aim of the present study was to evaluate the mechanisms of recurrent haemoptysis.
Methodology
• retrospective
• observational
• performed at one institution
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ryuge, M., Hara, M., Hiroe, T. et al. Mechanisms of recurrent haemoptysis after super-selective bronchial artery coil embolisation: a single-centre retrospective observational study. Eur Radiol 29, 707–715 (2019). https://doi.org/10.1007/s00330-018-5637-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5637-2