Abstract
Objectives
To evaluate the ability of different MRI sequences to detect chondrocalcinosis within knee cartilage and menisci, and to analyze the association with joint degeneration.
Methods
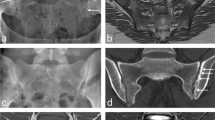
Subjects with radiographic knee chondrocalcinosis (n = 90, age 67.7 ± 7.3 years, 50 women) were selected from the Osteoarthritis Initiative and matched to controls without radiographic chondrocalcinosis (n = 90). Visualization of calcium-containing crystals (CaC) was compared between 3D T1-weighted gradient-echo (T1GE), 3D dual echo steady-state (DESS), 2D intermediate-weighted (IW), and proton density (PD)-weighted fast spin-echo (FSE) sequences obtained with 3T MRI and correlated with a semiquantitative CaC score obtained from radiographs. Structural abnormalities were assessed using Whole-Organ MRI Score (WORMS) and logistic regression models were used to compare cartilage compartments with and without CaC.
Results
Correlations between CaC counts of MRI sequences and degree of radiographic calcifications were highest for GE (rT1GE = 0.73, P < 0.001; rDESS = 0.68, P < 0.001) compared to other sequences (P > 0.05). Meniscus WORMS was significantly higher in subjects with chondrocalcinosis compared to controls (P = 0.005). Cartilage defects were significantly more frequent in compartments with CaC than without (patella: P = 0.006; lateral tibia: P < 0.001; lateral femur condyle: P = 0.017).
Conclusions
Gradient-echo sequences were most useful for the detection of chondrocalcinosis and presence of CaC was associated with higher prevalence of cartilage and meniscal damage.
Key Points
• Magnetic resonance imaging is useful for assessing burden of calcium-containing crystals (CaC).
• Gradient-echo sequences are superior to fast spin echo sequences for CaC imaging.
• Presence of CaC is associated with meniscus and cartilage degradation.





Similar content being viewed by others
Abbreviations
- BMEP:
-
Bone marrow edema pattern
- BCP:
-
Basic calcium phosphate
- CaC:
-
Calcium-containing crystal
- CPPD:
-
Calcium pyrophosphate deposition
- DESS:
-
Dual echo steady-state
- FSE:
-
Fast spin echo
- GE:
-
Gradient echo
- ICC:
-
Intra-class correlation coefficients
- KL:
-
Kellgren–Lawrence
- OA:
-
Osteoarthritis
- OAI:
-
Osteoarthritis Initiative
- WORMS:
-
Whole-Organ Magnetic Resonance Imaging Score
References
Ea HK, Liote F (2009) Advances in understanding calcium-containing crystal disease. Curr Opin Rheumatol 21:150–157
Wise CM (2007) Crystal-associated arthritis in the elderly. Rheum Dis Clin North Am 33:33–55
Mitsuyama H, Healey RM, Terkeltaub RA, Coutts RD, Amiel D (2007) Calcification of human articular knee cartilage is primarily an effect of aging rather than osteoarthritis. Osteoarthritis Cartilage 15:559–565
Neogi T, Nevitt M, Niu J et al (2006) Lack of association between chondrocalcinosis and increased risk of cartilage loss in knees with osteoarthritis: results of two prospective longitudinal magnetic resonance imaging studies. Arthritis Rheum 54:1822–1828
Ea HK, Nguyen C, Bazin D et al (2011) Articular cartilage calcification in osteoarthritis: insights into crystal-induced stress. Arthritis Rheum 63:10–18
Nowatzky J, Howard R, Pillinger MH, Krasnokutsky S (2010) The role of uric acid and other crystals in osteoarthritis. Curr Rheumatol Rep 12:142–148
Fuerst M, Bertrand J, Lammers L et al (2009) Calcification of articular cartilage in human osteoarthritis. Arthritis Rheum 60:2694–2703
Sun Y, Mauerhan DR, Honeycutt PR et al (2010) Calcium deposition in osteoarthritic meniscus and meniscal cell culture. Arthritis Res Ther 12:R56
Jubeck B, Gohr C, Fahey M et al (2008) Promotion of articular cartilage matrix vesicle mineralization by type I collagen. Arthritis Rheum 58:2809–2817
Steinbach LS (2004) Calcium pyrophosphate dihydrate and calcium hydroxyapatite crystal deposition diseases: imaging perspectives. Radiol Clin North Am 42:185–205, vii
Misra D, Guermazi A, Sieren JP et al (2015) CT imaging for evaluation of calcium crystal deposition in the knee: initial experience from the Multicenter Osteoarthritis (MOST) study. Osteoarthritis Cartilage 23:244–248
Beltran J, Marty-Delfaut E, Bencardino J et al (1998) Chondrocalcinosis of the hyaline cartilage of the knee: MRI manifestations. Skeletal Radiol 27:369–374
Suan JC, Chhem RK, Gati JS, Norley CJ, Holdsworth DW (2005) 4 T MRI of chondrocalcinosis in combination with three-dimensional CT, radiography, and arthroscopy: a report of three cases. Skeletal Radiol 34:714–721
Checa A, Chun W (2015) Rates of meniscal tearing in patients with chondrocalcinosis. Clin Rheumatol 34:573–577
Lefevre N, Naouri JF, Herman S, Gerometta A, Klouche S, Bohu Y (2016) A current review of the meniscus imaging: proposition of a useful tool for its radiologic analysis. Radiol Res Pract 2016:25
Wadhwa V, Cho G, Moore D, Pezeshk P, Coyner K, Chhabra A (2016) T2 black lesions on routine knee MRI: differential considerations. Eur Radiol 26:2387–2399
Peterfy C, Li J, Zaim S et al (2003) Comparison of fixed-flexion positioning with fluoroscopic semi-flexed positioning for quantifying radiographic joint-space width in the knee: test-retest reproducibility. Skeletal Radiol 32:128–132
Felson DT, Nevitt MC, Yang M et al (2008) A new approach yields high rates of radiographic progression in knee osteoarthritis. J Rheumatol 35:2047–2054
Smith HE, Mosher TJ, Dardzinski BJ et al (2001) Spatial variation in cartilage T2 of the knee. J Magn Reson Imaging 14:50–55
Peterfy CG, Schneider E, Nevitt M (2008) The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage 16:1433–1441
Peterfy CG, Guermazi A, Zaim S et al (2004) Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage 12:177–190
Stehling C, Lane NE, Nevitt MC, Lynch J, McCulloch CE, Link TM (2010) Subjects with higher physical activity levels have more severe focal knee lesions diagnosed with 3T MRI: analysis of a non-symptomatic cohort of the osteoarthritis initiative. Osteoarthritis Cartilage 18:776–786
Bucknor MD, Nardo L, Joseph GB et al (2015) Association of cartilage degeneration with four year weight gain- 3T MRI data from the osteoarthritis initiative. Osteoarthritis Cartilage. doi:10.1016/j.joca.2014.10.013
Kaushik S, Erickson JK, Palmer WE, Winalski CS, Kilpatrick SJ, Weissman BN (2001) Effect of chondrocalcinosis on the MR imaging of knee menisci. AJR Am J Roentgenol 177:905–909
Jungmann PM, Nevitt MC, Baum T et al (2015) Relationship of unilateral total hip arthroplasty (THA) to contralateral and ipsilateral knee joint degeneration - a longitudinal 3T MRI study from the Osteoarthritis Initiative (OAI). Osteoarthritis Cartilage 23:1144–1153
Stehling C, Liebl H, Krug R et al (2010) Patellar cartilage: T2 values and morphologic abnormalities at 3.0-T MR imaging in relation to physical activity in asymptomatic subjects from the osteoarthritis initiative. Radiology 254:509–520
Kretzschmar M, Lin W, Nardo L et al (2015) Association of physical activity measured by accelerometer, knee joint abnormalities and cartilage T2-measurements obtained from 3T MRI: data from the osteoarthritis initiative. Arthritis Care Res (Hoboken). doi:10.1002/acr.22586
Riederer I, Karampinos DC, Settles M et al (2015) Double inversion recovery sequence of the cervical spinal cord in multiple sclerosis and related inflammatory diseases. AJNR Am J Neuroradiol 36:219–225
Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW (1988) Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 15:1833–1840
Lin LI (1989) A concordance correlation coefficient to evaluate reproducibility. Biometrics 45:255–268
Baum T, Joseph GB, Arulanandan A et al (2012) Association of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with knee pain: data from the osteoarthritis initiative. Arthritis Care Res (Hoboken) 64:248–255
Baum T, Stehling C, Joseph GB et al (2012) Changes in knee cartilage T2 values over 24 months in subjects with and without risk factors for knee osteoarthritis and their association with focal knee lesions at baseline: data from the osteoarthritis initiative. J Magn Reson Imaging 35:370–378
Pan J, Pialat JB, Joseph T et al (2011) Knee cartilage T2 characteristics and evolution in relation to morphologic abnormalities detected at 3-T MR imaging: a longitudinal study of the normal control cohort from the osteoarthritis initiative. Radiology 261:507–515
Abreu M, Johnson K, Chung CB et al (2004) Calcification in calcium pyrophosphate dihydrate (CPPD) crystalline deposits in the knee: anatomic, radiographic, MR imaging, and histologic study in cadavers. Skeletal Radiol 33:392–398
Bloecker K, Wirth W, Guermazi A, Hitzl W, Hunter DJ, Eckstein F (2015) Longitudinal change in quantitative meniscus measurements in knee osteoarthritis--data from the osteoarthritis initiative. Eur Radiol 25:2960–2968
Eckstein F, Boudreau R, Wang Z et al (2016) Comparison of radiographic joint space width and magnetic resonance imaging for prediction of knee replacement: a longitudinal case-control study from the osteoarthritis initiative. Eur Radiol 26:1942–1951
Acknowledgments
The scientific guarantor of this publication is Dr. Thomas M. Link, MD, PhD, Department of Radiology and Biomedical Imaging, University of California, San Francisco. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. Written informed consent was obtained from all subjects in this study. Institutional Review Board approval was obtained. This manuscript was prepared using an OAI public use data set and has received the approval of the OAI Publications Committee based on a review of its scientific content and data interpretation. The analyses in this study were funded through the NIH (National Institute of Arthritis and Musculoskeletal and Skin Diseases grants R01AR064771 and P50-AR060752).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gersing, A.S., Schwaiger, B.J., Heilmeier, U. et al. Evaluation of Chondrocalcinosis and Associated Knee Joint Degeneration Using MR Imaging: Data from the Osteoarthritis Initiative. Eur Radiol 27, 2497–2506 (2017). https://doi.org/10.1007/s00330-016-4608-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4608-8