Abstract
Objective
The purpose of this study was to evaluate two MR-guided biopsy techniques at 3 T, large core needle breast biopsy (LCNB) and vacuum-assisted breast biopsy (VAB) and to compare the diagnostic yield and rate of complications to determine the optimal biopsy technique at 3 T.
Methods
55 LCNB and 64 VAB were consecutively performed. Benign biopsy results were verified by retrospective correlation of histology, with pre-interventional, post-interventional MRI studies and follow-up and were classified as representative or non-representative. Time to follow-up was up to 2 years for the considered non-representative benign lesions. Statistical analysis was performed using the Chi-squared test.
Results
LCNB was technically successful in 100% of patients (55/55) and VAB in 98% of patients (63/64). Histopathological analysis resulted in 45 (82%) benign, 3 (5%) high-risk and 7 (13%) malignant lesions for LCNB and 43 (67%) benign, 3 (5%) high-risk and 18 (28%) malignant lesions. Distribution was significantly different (p < 0.001), favouring VAB over LCNB.
Conclusion
Because of the substantially higher diagnostic yield and certainty of a benign diagnosis, VAB is the optimal biopsy technique at 3 T. LCNB should be considered when VAB is not feasible.
Similar content being viewed by others
Introduction
The use of Magnetic Resonance Imaging (MRI) for the detection and evaluation of breast lesions continues to increase. MRI of the breast has high sensitivity and lower specificity in the evaluation of breast lesions, with sensitivity of 90% and a specificity of 72% [1].
The increasing use of magnets with high field strength (3 Tesla [T] and over) in clinical practice is very suitable. With the high signal-to-noise ratio of these systems, appropriate acquisitions can be used to achieve high spatial and temporal resolution that allows depiction of lesion morphology and lesion enhancement with unprecedented accuracy [2–4]. Compared with the results of 1 T and 1.5 T MRI in the literature, 3 T may have an even higher sensitivity to breast cancer [5]. Nevertheless, even at 3 T it is often not possible to separate malignant from benign findings purely on imaging characteristics alone. Accurate diagnosis of these lesions requires histological evaluation.
Magnetic resonance imaging of the breast reveals suspicious lesions occult at target ultrasound or mammography in about 44% of the cases [6, 7]. In these suspicious MRI breast lesions MRI-guided biopsy is the technique of choice. Moreover, MRI-guided biopsy is indicated in all lesions where an ultrasound or mammographically detected and biopsied lesion is possibly not the same lesion as that detected at MRI. The demand for MRI-guided breast interventions is growing owing to the increasing use of breast MRI in clinical practice.
Magnetic resonance-guided tissue sampling of suspicious MRI breast lesions, can be accomplished by fine-needle aspiration, wire localisation followed by surgical excision, large core-needle biopsy (LCNB), or by vacuum-assisted biopsy (VAB) [8, 9].
Magnetic resonance-guided LCNB using 14 G needles and VAB have both been described as suitable techniques for MRI-guided breast biopsy at 1.5 T [10–21]. At 3 T only MRI-guided LCNB has been evaluated [22, 23]. Moreover, no studies directly compare the diagnostic yield of LCNB with VAB at any field strength. In this retrospective study we evaluated both methods at 3 T and compared the diagnostic yield and rate of complications to determine the optimal biopsy technique at 3 T.
Materials and methods
Patient characteristics
Patients derive from a diagnostic population, who presented with an MRI-detected lesion between July 2007 and February 2010, not visualised at second-look ultrasound or in whom second-look ultrasound could not reliably reproduce the lesion, were scheduled for MRI-guided biopsy at 3 T. Before October 2008 14 G LCNB were performed, thereafter 9 G VAB was the principal method. We retrospectively analysed the data from a total of 147 consecutive women (mean age; range: 51; 29–71 years) with 155 suspicious breast lesions at our hospital, in this period. Indications for breast MRI are shown in Table 1. Patients were referred for MRI-guided biopsy from 22 different hospitals. Patients with a breast MRI from a different hospital, had a second reading from a MRI-breast radiologist in our institution, before the biopsy. In doubtful cases an additional diagnostic breast-MRI was performed in our institution.
The level of suspicion was reported on a scale of 0–6, identical to that in the lesion-assessment categories used in the breast imaging reporting and data system (BI-RADS) [24]. Numeric categories were the following: 0, needs additional imaging evaluation; 1, normal; 2, benign; 3, probably benign; 4, suspicious; 5, highly suggestive of malignancy; 6 proven malignancy. Classification was based on lesion morphology and enhancement kinetics [25]. For lesions interpreted as probably benign, suspicious or highly suggestive of malignancy on MRI, correlative ultrasound was performed to determine if the lesion was ultrasonographically evident and, thereby amenable to tissue sampling under ultrasound guidance. All patients with a BIRADS 3, 4 or 5 lesions that were ultrasonographically occult were scheduled for a MRI-guided breast biopsy. In 80% of the biopsied cases there was a mass lesion, in 13% of the biopsied cases there was non mass like enhancement and in 7% of the cases there was a focus.
All MRI-guided breast biopsies were performed in a clinical setting. The study has been carried out in the Netherlands in accordance with the applicable rules concerning the review of research ethics committees and informed consent.
MRI-guided biopsy procedure and equipment
All biopsies were performed on a Siemens Magnetom Trio system, using a dedicated four-channel open breast coil (Invivo Interventional Instruments, Wurzburg, Germany), with an add-on device for needle positioning (Noras, Germany).
Patients were positioned on the MRI table in the prone position with the affected breast compressed in the biopsy device to avoid motion during the biopsy procedure. All patients underwent imaging before and three times after the administration of 0.1 mmol/kg contrast agent (DOTAREM, Guerbet, France), using a T1-weighted Fast Low Angle Shot (FLASH) 3D acquisition that lasted 98 s and provided coverage of both breasts with near isotropic voxels of 0.9*0.9*1 mm to allow image reconstruction in any plane. The main parameters of this MRI sequence are listed in Table 2. A dedicated computer program (DynaCAD, Invivo, Germany) was used to obtain coordinates of the lesion and guidance for needle positioning (Figs. 1 and 2). After initial imaging, the table was moved out of the bore and local anaesthesia (lidocaine 1%) was administered after disinfection of the breast. A small incision was made in the skin and the needle (LCNB or VAB) was inserted into the breast. The position of the device was evaluated with a FLASH 3D and another FLASH 3D was obtained immediately post-biopsy to correlate the biopsy cavity (at LCNB) and the marker (at VAB, ATEC TriMark, Indianapolis, IN, USA) with the position of the lesion.
Screenshot from a biopsy procedure, using a 14 G large core needle to biopsy an non-mass-like area of enhancement in the upper outer quadrant of the left breast. The lower screen shows how to position the post and pillar device. The coordinates for optimal needle position can be read from the bottom of the screen
Screenshot from a biopsy procedure using a 9 G vacuum-assisted needle to biopsy a small mass-like lesion with an irregular margin at six o’clock in the left breast. The lower screen shows where to put the needle block (purple square) and which hole to use (orange dot) in order to come closest to the optimal position (red circle). The required depth can be read at the bottom of the screen (arrow)
MRI-guided large core needle biopsy
Large core-needle biopsy was performed with 14-gauge titanium needles using a post and pillar system that allowed angulation of the biopsy tract up to 30° (Fig. 3). Through a sterile 12-gauge needle holder a 12-gauge coaxial needle was inserted in the breast (CoaxNeedle Highfield 12 G-11.1 mm; Invivo Interventional Instruments). In the event of good correlation the patient was moved out of the bore of the magnet. Between 5 and 6 biopsies were taken with a disposable 14 G semi-automatic Highfield MRI compatible biopsy gun (Invivo Interventional Instruments).
Post and pillar biopsy system with LCB system in place, note that the needle is angulated towards the thoracic wall. The blue device at the post and pillar system is a needle sleeve that can be used to lock the co-axial needle in one position when the biopsy needle is inserted and removed to obtain multiple samples
MRI-guided vacuum-assisted biopsy
Vacuum-assisted biopsy were performed with one of two commercially available MRI-compatible VAB devices: either a 10-gauge VAB system (SenorRx; Irvine, CA, USA) or a 9-gauge VAB system (ATEC Suros, Indianapolis, IN, USA). We used a grid system with a needle block as supplied by the manufacturer; this approach did not allow angulation (Fig. 4).
A coaxial sheath was placed using a 9-gauge or 10-gauge titanium stylet in the appropriate grid location. The stylet was replaced by a sterile plastic MRI-visible obturator. If a FLASH 3D proved a good correlation, the plastic obturator was replaced by one of the respective VAB devices and up to 12 biopsies were taken.
Data collection and statistic analysis
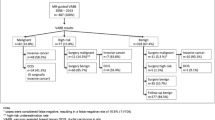
Of the 147 patients with suspicious breast lesions, 4 patients had two lesions and 2 patients had three lesions. Thirteen lesions were biopsied twice with the same technique VAB or LCNB. A total of 168 suspected lesions were scheduled for an MRI-guided biopsy, 41 BIRADS 3 lesions, 111 BIRADS 4 lesions and 16 BIRADS 5 lesions. In 26 patients the procedure was cancelled. Eighteen biopsies were excluded because they were judged as not representative lesions after histopathologic analysis, an extra 4 biopsies were excluded because of losses to follow-up and 1 biopsy was excluded because of technical failure. Finally we included 55 LCNB and 64 VAB in a consecutive series of patients scheduled for MRI-guided breast biopsy (Fig. 5). Of the 64 VAB, 35 biopsies were performed with the 10-gauge VAB system (SenorRx) and the other 29 biopsies were performed with a 9-gauge VAB system (ATEC Suros).
All biopsies were performed by three experienced MRI-breast radiologists who had a lot of experience with MRI-guided LCNB and less with MRI-guided VAB. All three radiologist had more than 3 years experience with MRI-guided LCNB and had done more than 50 procedures each. MRI-guided VAB was only done at 1.5 T for 1 year and all three radiologist had done about 10 procedures each, before they started to do the same procedure at 3 T.
We assessed technical success rates, where technical success was defined as:
-
1.
It is possible to get the needle on the right spot
-
2.
The biopsy can be safely performed according to the performing physician
-
3.
The lesion was removed completely or partially according to the post-VAB imaging.
We judged VAB not possible, if the risk of skin tears, severe bleeding, or severe pain in the patient was high. Complications, such as pain and arterial bleeds were registered and were in this study not considered as technical unsuccessful.
After histopathological analysis, the lesions were classified in three categories: benign, high-risk or malignant. Malignant lesions and high-risk lesions were surgically removed. In the case of a benign lesion, the images of the biopsy procedure were re-evaluated to determine whether the tissue samples were representative and at least 6-month follow-up MRI was recommended. Total time to follow-up was 2 years in case of unchanged lesions.
Statistical analysis was done with SPSS 12.0 and using the Mann–Whitney U test. Significance was assumed at a level of p < 0.05.
Results
Technical success
In our patient series, LCNB was technically successful in 100% of patients (55/55) and VAB was technically successful in 98% of patients (63/64) (n.s.).
According to the post-interventional imaging 1 of the 72 VAB procedures was unsuccessful and immediate rebiopsy was performed. Assessment was considered impaired, but successful in 1 LCNB procedure and 3 VAB procedures (n.s.). Motion artefacts occurred in 1 patient at LCNB. Histological analysis of the lesion revealed normal breast tissue. After more than 1 year of follow-up, no signs of malignancy were found.
Using VAB 2 small arterial haemorrhages that required prolonged manual compression were recorded (Fig. 6). In one patient no marker could be placed because the patient could no longer lie in the prone position.
Biopsy of a small irregular lesion at 6 o’clock in the left breast using a 9 G vacuum-assisted needle. a subtraction image created before the biopsy procedure to localise the lesion. b placement of the coaxial sheet, the trocar has been removed and replaced with a plastic insert. c the same coaxial sheet directly after the biopsy, note the large haematoma that surrounds the tip of the needle (double-headed arrow). d A large marker is left that also provides some compression from the inside. Histopathological results revealed a complex sclerosing lesion with cystic degeneration. No malignancy was found
The lesion size ranged from 3 to 28 mm with a median lesion size of 12 mm, 50% were smaller than 1 cm (28 LCNB and 32 VAB) and 8% of all the lesions were smaller than 5 mm (foci) and were all biopsied with MRI-guided VAB.
Clinical results
Final histology results obtained in 55 LCNB and 64 VAB procedures are listed in Table 3.
In the LCNB group histopathological analysis of the biopsy samples resulted in benign lesions in 45/55 (82%), in high-risk lesions in 3/55 (5%) and in malignant lesions in 7/55 (13%). In the VAB group this resulted in 43/64 (67%) benign lesions, 3/64 (5%) and 18/64 (28%) malignant lesions (Fig. 5).
Discussion
We evaluated LCNB and VAB using a 3 T clinical MR system and compared the diagnostic yield and rate of complications to determine the optimal biopsy technique at 3 T.
Published data on the use of MRI-guided biopsy at 1.5 T reported a technical success rate of 95% to 100% and cancer yields ranging from 20% to 56% for LCNB [10, 11, 15], and a technical success rate of 96% to 100% and cancer yields ranging from 24% to 40% for VAB [16–21]. These numbers are comparable to the accepted rates reported in mammography- and ultrasound-guided procedures [8]. In our patient series, in the LCNB group 13% were found to be malignant after biopsy and 28% in the VAB group. LCNB was technically successful in 100% of patients (55/55) and VAB was technically successful in 98% of patients (63/64), but more importantly VAB was significantly more often representative than LCNB.
Magnetic resonance-guided LCNB using 14 G needles at 1.5 T have been reported by several groups [10–15]. Kuhl et al. [10] reported the largest series of MRI-guided LCNB in 59 patients with 78 lesions. One carcinoma was missed, and histological underestimations were not observed. Chen et al. [11] reported 34 successful LCNB in 35 lesions in 29 women and 6% (2/35) were underestimated carcinoma. Two lesions with atypical ductal hyperplasia were upgraded to malignancy after surgery. In a multicentre European trial Perlet et al. [18] reported successful VAB in 517 (96%) of 538 lesions. Histological underestimation was observed in 8 patients (2%). An upgrade was noted in 5 patients who had a diagnosis of ADH, and in 3 patients who had a diagnosis of DCIS.
The biopsies we performed, 20% of the LCNB were considered unrepresentative biopsies and were excluded from this study. After chirurgical excision biopsy 7% of carcinomas were missed and histological underestimation was present in 6%. VAB showed 4 unrepresentative biopsies, 2 missed carcinomas and histological underestimation was not observed. Eight of the 14 non-representative LCNB were smaller than 1 cm (57%). Therefore, LCNB biopsy at 3 T is not recommended for lesions smaller than 10 mm.
Two of our VAB procedures were converted to LCNB because of the location of the lesion (too close to the skin and the other lesion was too close to the chest wall), which is in part attributable to the more rigid biopsy technique with the grid system. Using angulation of the needle will probably allow VAB in a number of patients in whom the technique first failed. In another patient scheduled for LCNB a 14 G needle could not enter the breast because of the solid consistency of the breast. Finally a successful biopsy was performed with an 18 G needle.
The chance of detecting malignancy is very much dependent on the à priori chance of the biopsied lesion being malignant. It is important to realise that it is essential to compare the results of the pathological analysis of the biopsy specimen with the lesion seen at MRI. A diagnosis of fibrosis with chronic inflammation is usually acceptable in lesions with no mass effect classified as BI-RADS 3 to BI-RADS 4a. However, this diagnosis is absolutely insufficient in lesions classified as BI-RADS 5, as well as in any lesion presenting as a clear mass.
For BIRADS III (probably benign) lesions, at mammography a 6-month short-term follow-up is recommended, because the frequency of carcinoma in these lesions is low, ranging from 0.5% to 2% [26–29]. Published results show a wide range of cancer yields (0.6% to 10%) among probably benign MRI lesions [30–35]. There are a few indications for which mammographic stereotactic biopsy of BIRADS III lesions may be considered, such as when follow-up is compromised or not available, in the instance of planned pregnancy, or for patient anxiety [27, 36–38]. Eighteen percent (41 lesions) of all the MRI-guided biopsied lesions were classified as BIRADS III breast lesions, because a lot of women nowadays do not except a 6 month follow-up and prefer a biopsy directly. Three percent of these lesions were found malignant at histopathologic analysis.
Magnetic resonance-guided LCNB using 14 G needles and VAB have both been described as suitable techniques for MRI-guided breast biopsy at 1.5 T [8, 9], although several potential limitations of LCNB have been described at 1.5 T or lower [9]. Compared with 1.5 T, at 3 T an extra potential limitation of the LCNB technique has been described by Peters et al. [22]. Susceptibility artefacts increase with increasing field strength. This can cause a substantial signal void around the 14 G needle when performing MRI-guided LCNB at 3 T.
The certainty of the histopathological diagnosis increases with an increase in the amount of tissue extracted, especially in a situation where there is no real-time feedback of the needle position during biopsy, as is the case in MRI-guided breast biopsy. In lesions with no mass effect LCNB may not sample enough material to ascertain diagnosis. In mass lesions the non-rigid breast tissue may allow a small solid lesion to move during biopsy, causing the needle to pass right beside the lesion, rather than through its core.
Our study has several limitations. First of all in our study population most patients with a representative benign lesion had a 6 month follow-up instead of a 24 months follow up which is necessary to make them eligible to be considered as truly benign.
An important limitation of this study is the fact that the two biopsy techniques were compared in a sequential and not in a parallel way. Lots of covariates could influence the results of the two study arms, the most important being the fact that experience was much higher for the second biopsy technique, resulting in a much steeper learning curve and possibly better results. Also the fact that two different vacuum assisted biopsy devices have been used is another potential limitation as the study subgroup becomes heterogeneous.
In our study up to 12 VAB were taken instead of >24 core (11-Gauge) specimens, which is recommended in the latest European consensus meeting [39].
Another very important limitation is the highly heterogeneous study population. The fact that patients were referred from 22 different hospitals for MRI-guided biopsy and the consecutively study population is a major limitation. Although all breast MRI’s from the different hospitals, had a second reading from a MRI-breast radiologist in our institution, before the biopsy and in doubtful cases an additional diagnostic breast MRI was made in our institution, it is unthinkable that equal clinical guidelines for such referrals apply for all hospitals or that experience levels match for all those different radiologists.
Finally, by the time we started with MRI-guided LCNB at 3 T, large core needles with 14 G were not anymore recommended for MRI-guided biopsy at 1.5 T [8, 9].
Compared with the results of 1 T and 1.5 T MRI in the literature, 3 T may have higher sensitivity in the detection of breast cancer [5]. Nevertheless the specificity is comparable with the results of 1.5 T [5]. Demand for adequate MRI-guided tissue sampling remains, also at high field strength. Our study shows that MRI-guided VAB should be used for all MRI-guided breast biopsies at 3 T because of the substantially higher diagnostic yield and increased certainty of a benign diagnosis, compared with LCNB. LCNB should be considered when VAB is unavailable or not feasible.
References
Peters NH, Borel Rinkes IH, Zuithoff NP et al (2008) Meta-analysis of MR imaging in the diagnosis of breast lesions. Radiology 246:116–124
Kuhl CK, Jost P, Morakkabati N et al (2006) Contrast-enhanced MR imaging of the breast at 3.0 and 1.5 T in the same patients: initial experience. Radiology 239:666–676
Rakow-Penner R, Daniel B, Yu H et al (2006) Relaxation times of breast tissue at 1.5 T and 3 T measured using IDEAL. J Magn Reson Imag 23:87–91
Sasaki M, Shibata E, Kanbara Y et al (2005) Enhancement effects and relaxivities of gadolinium-DTPA at 1.5 versus 3 Tesla: a phantom study. Magn Reson Med Sci 4:145–149
Elsamaloty H, Elzawawi MS, Mohammad S et al (2009) Increasing accuracy of detection of breast cancer with 3-T MRI. AJR Am J Roentgenol 192:1142–1148
Abe H, Schmidt RA, Shah RN, Shimauchi A et al (2010) MR-directed (“second-look”) ultrasound examination for breast lesions detected initially on MRI: MR and sonographic findings. AJR Am J Roentgenol 194:370–377
Meissnitzer M, Dershaw DD, Lee CH et al (2009) Targeted ultrasound of the breast in women with abnormal MRI findings for whom biopsy has been recommended. AJR Am J Roentgenol 193:1025–1029
Eby PR, Lehman CD (2008) Magnetic resonance imaging-guided breast interventions. Top Magn Reson Imag 19:151–162
Floery D, Helbich TH (2006) MRI-guided percutaneous biopsy of breast lesions: materials, techniques, success rates, and management in patients with suspected radiologic-pathologic mismatch. Magn Reson Imag Clin N Am 14:411–425
Kuhl CK, Morakkabati N, Leutner CC et al (2001) MR imaging-guided large-core (14-gauge) needle biopsy of small lesions visible at breast MR imaging alone. Radiology 220:31–39
Chen X, Lehman CD, Dee KE (2004) MRI-guided breast biopsy: clinical experience with 14-gauge stainless steel core biopsy needle. AJR Am J Roentgenol 182:1075–1080
Lehman CD, Eby PR, Chen X et al (2003) MR imaging guided breast biopsy using a coaxial technique with a 14-gauge stainless steel core biopsy needle and a titanium sheath. AJR Am J Roentgenol 181:183–185
Fischer U, Kopka L, Grabbe E (1998) Magnetic resonance guided localization and biopsy of suspicious breast lesions. Top Magn Reson Imag 9:44–59
Kuhl CK, Elevelt A, Leutner CC et al (1997) Interventional breast MR imaging: clinical use of a stereotactic localization and biopsy device. Radiology 204:667–675
Pfleiderer SO, Reichenbach JR, Azhari T et al (2003) A manipulator system for 14-gauge large core breast biopsies inside a high-field whole-body MR scanner. J Magn Reson Imag 17:493–498
Orel SG, Rosen M, Mies C, Schnall MD (2006) MR imaging-guided 9-gauge vacuum-assisted core-needle breast biopsy: initial experience. Radiology 238:54–61
Liberman LBN (2005) MRI-guided 9-gauge vacuum assisted breast biopsy: initial clinical experience. AJR Am J Roentgenol 185:183–193
Perlet C, Heinig A, Prat X et al (2002) Multicenter study for the evaluation of a dedicated biopsy device for MR-guided vacuum biopsy of the breast. Eur Radiol 12:1463–1470
Liberman L, Morris EA, Dershaw DD et al (2003) Fast MRI-guided vacuum-assisted breast biopsy: initial experience. AJR Am J Roentgenol 181:1283–1293
Lehman CD, Deperi ER, Peacock S et al (2005) Clinical experience with MRI-guided vacuum-assisted breast biopsy. AJR Am J Roentgenol 184:1782–1787
Schrading S, Simon B, Braun M et al (2010) MRI-guided breast biopsy: influence of choice of vacuum biopsy system on the mode of biopsy of MRI-only suspicious breast lesions. AJR Am J Roentgenol 194:1650–1657
Peters NH, Meeuwis C, Bakker CJ et al (2009) Feasibility of MRI-guided large-core-needle biopsy of suspicious breast lesions at 3 T. Eur Radiol 19:1639–1644
Meeuwis C, Mann RM, Mus RD et al (2010) MRI-guided breast biopsy at 3 T using a dedicated large core biopsy set: feasibility and initial results. Eur J Radiol 79:257–261
Ikeda DM, Hylton NM, Kinkel K et al (2001) Development, standardization, and testing of a lexicon for reporting contrast-enhanced breast magnetic resonance imaging studies. J Magn Reson Imag 13:889–895
Kuhl CK (2000) MRI of breast tumors. Eur Radiol 10:46–58
Sickles EA, Parker SH (1993) Appropriate role of core breast biopsy in the management of probably benign lesions. Radiology 188:315
Graf O, Helbich TH, Fuchsjaeger MH et al (2004) Follow-up of palpable circumscribed noncalcified solid breast masses at mammography and US: can biopsy be averted? Radiology 233:850–856
Sickles EA (1999) Probably benign breast lesions: when should follow-up be recommended and what is the optimal follow- follow-up protocol? Radiology 213:11–14
Brenner RJ, Sickles EA (1997) Surveillance mammography and stereotactic core breast biopsy for probably benign lesion: a cost comparison analysis. Acad Radiol 4:419–425
Kriege M, Brekelmans CT, Boetes C et al (2004) Magnetic Resonance Imaging Screening Study Group Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med 351:427–437
Eby PR, DeMartini WB, Peacock S, Rosen EL, Lauro B, Lehman CD (2007) Cancer yield of probably benign breast MR examinations. J Magn Reson Imag 26:950–955
Sadowski EA, Kelcz F (2005) Frequency of malignancy in lesions classified as probably benign after dynamic contrast-enhanced breast MRI examination. J Magn Reson Imag 21:556–564
Liberman L, Morris EA, Benton CL, Abramson AF, Dershaw DD (2003) Probably benign lesions at breast magnetic resonance imaging: preliminary experience in high-risk women. Cancer 98:377–388
Kuhl CK, Schmutzler RK, Leutner CC et al (2000) Breast MR imaging screening in 192 women proved or suspected to be carriers of a breast cancer susceptibility gene: preliminary results. Radiology 215:267–279
Eby PR, DeMartini WB, Gutierrez RL, Saini MH, Sue Peacock S, Lehman CD (2009) Characteristics of probably benign breast MRI lesions. AJR 193:861–867
Helbich TH, Matzek W, Fuchsjager MH (2004) Stereotactic and ultrasound-guided breast biopsy. Eur Radiol 14:383–393
Liberman L (2000) Centennial dissertation. Percutaneous imaging-guided core breast biopsy: state of the art at the millennium. AJR Am J Roentgenol 174:1191–1199
Vizcaino I, Gadea L, Andreo L et al (2001) Short-term follow-up results in 795 nonpalpable probably benign lesions detected at screening mammography. Radiology 219:475–483
Heywang-Köbrunner SH, Sinnatamby R, Lebeau A et al (2009) Interdisciplinary consensus on the uses and technique of MR-guided vacuum-assisted breast biopsy (VAB): results of a European consensus meeting. Eur J Radiol 72:289–294
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Carla Boetes deceased
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Meeuwis, C., Veltman, J., van Hall, H.N. et al. MR-guided breast biopsy at 3T: diagnostic yield of large core needle biopsy compared with vacuum-assisted biopsy. Eur Radiol 22, 341–349 (2012). https://doi.org/10.1007/s00330-011-2272-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-011-2272-6