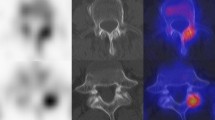
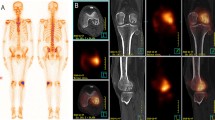
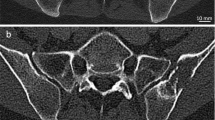
Abstract
Current imaging guidelines recommend that many cancer patients undergo soft-tissue staging by computed tomography (CT) whilst the bones are imaged by skeletal scintigraphy (bone scan). New CT technology has now made it feasible, for the first time, to perform a detailed whole-body skeletal CT. This advancement could save patients from having to undergo duplicate investigations. Forty-three patients with known malignancy were investigated for bone metastasis using skeletal scintigraphy and 16-detector multislice CT. Both studies were performed within six weeks of each other. Whole-body images were taken 4 h after injection of 500 Mbq 99mTc-MDP using a gamma camera. CT was performed on a 16-detector multislice CT machine from the vertex to the knee. The examinations were reported independently and discordant results were compared at follow-up. Statistical equivalence between the two techniques was tested using the Newcombe-Wilson method within the pre-specified equivalence limits of ±20%. Scintigraphy detected bone metastases in 14/43 and CT in 13/43 patients. There were seven discordances; four cases were positive on scintigraphy, but negative on CT; three cases were positive on CT and negative on scintigraphy. There was equivalence between scintigraphy and CT in detecting bone metastases within ±19% equivalence limits. Patients who have undergone full whole-body staging on 16-detector CT may not need additional skeletal scintigraphy. This should shorten the cancer patient's diagnostic pathway.




Similar content being viewed by others
References
Royal College of Radiologists (2003) Making the best use of a department of clinical radiology; guidelines for doctors, 5th edn. RCR, London, UK
Medical Device Agency (2002) Eight and sixteen slice CT scanner comparison report. Medical Device Agency, MDA 02059, HMSO, Norwich, UK
Murray IPC (2004) Bone scintigraphy in trauma. In: Ell PJ, Gambhir SS (eds) Nuclear medicine in clinical diagnosis and treatment, 3rd edn. Church Livingstone, New York, pp 641–656
National Radiological Protection Board (1998) Notes for guidance on clinical administration of radiopharmaceuticals and use of sealed sources. Administration of Radioactive Substances Advisory Committee. National Radiological Protection Board, Chilton, UK
Savelli G, Maffioli L, Maccauro M, De Deckere E, Bombardieri E (2001) Bone scintigraphy and the added value of SPECT (single photon emission tomography) in detecting skeletal lesions. Q J Nucl Med 45:27–37
Lauenstein TC, Goehde SC, Herborn CU, Treder W, Ruehm SG, Debatin JF, Barkhausen J (2002) Three-dimensional volumetric interpolated breath-hold MR imaging for whole-body tumor staging in less than 15 minutes: a feasibility study. AJR Am J Roentgenol 179:445–449
Walker RE, Eustace SJ (2001) Whole-body magnetic resonance imaging: techniques, clinical indications, and future applications. Semin Musculoskelet Radiol 5:5–20
Eustace SJ, Nelson E (2004) Whole body magnetic resonance imaging. BMJ 328:1387–1388
Lauenstein TC, Goehde SC, Herborn CU, Goyen M, Oberhoff C, Debatin JF, Ruehm SG, Barkhausen J (2004) Whole-body MR imaging: evaluation of patients for metastases. Radiology 233:139–148
Cook GJR, Fogelman I (2000) The role of positron emission tomography in the management of bone metastases. Cancer 88:2927–2933
Uematsu T, Yuen S, Yukisawa S, Aramaki T, Morimoto N, Endo M, Furukawa H, Uchida Y, Watanabe J (2005) Comparison of FDG PET and SPECT for detection of bone metastases in breast cancer. AJR Am J Roentgenol 184:1266–1273
Hetzel M, Arslandemir C, Konig HH, Buck AK, Nussle K, Glatting G, Gabelmann A, Hetzel J, Hombach V, Schirrmeister H (2003) F-18 NaF PET for detection of bone metastases in lung cancer: accuracy, cost-effectiveness, and impact on patient management. J Bone Miner Res 18:2206–2214
Even-Sapir E, Metser U, Flusser G, Zuriel L, Kollender Y, Lerman H, Lievshitz G, Ron I, Mishani E (2004) Assessment of malignant skeletal disease: initial experience with 18F-fluoride PET/CT and comparison between 18F-fluoride PET and 18F-fluoride PET/CT. J Nucl Med 45:272–278
Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Lijmer JG, Moher D, Rennie D, de Vet HC, Standards for Reporting of Diagnostic Accuracy (2003) Towards complete and accurate reporting of studies of diagnostic accuracy: The STARD Initiative. Ann Intern Med 138:40–44
Newcombe RG (1998) Improved confidence intervals for the difference between binomial proportions based on paired data. Stat Med 17:2635–2650
Center for Drug Evaluation and Research (2001) Guidance for the industry. Statistical approaches to establishing bioequivalence. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER)
Newcombe RG (1988) Confidence interval for difference of proportions based on Newcombe-Wilson method. In: EquivTest from Statistical Solutions: software for statistical analysis of equivalence and bioavailability studies user reference. Statistical Solutions, Saugus, Massachusetts, pp 40–54
Farr RF, Allisy-Roberts PJ (1997) Gamma imaging. In: Farr RF, Allisy-Roberts PJ (eds) Physics for medical imaging, 1st edn. Saunders, London, pp 132
Medical Services Agency (2003) ImPACT CT patient dosimetry calculator version 0.99 q. Medical Devices Agency, London
Groves AM, Owen K, Courtney HM, Yates S, Goldstone KE, Blake GM, Dixon AK (2004) 16-detector multislice CT: estimation by TLD measurement compared with Monte Carlo simulation. Br J Radiol 77:662–665
Mountford PJ, O'Doherty MJ (1999) Exposure of critical groups to nuclear medicine patients. Appl Radiat Isot 50:89–111
McDonnell CE (1996) Assessment of the radiological consequences of accumulation and disposal of radioactive wastes by small users of radioactive materials. National Radiological Protection Board, Chilton, UK, report no NRPB-M744
Acknowledgements
The authors are indebted to all the technical/radiographic and clerical staff of both the nuclear medicine and radiology departments of our hospital. The authors appreciate our Institution’s Urology and Oncology doctors for cooperating with our study. The study received a small financial award from the Royal Society of Medicine, UK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Groves, A.M., Beadsmoore, C.J., Cheow, H.K. et al. Can 16-detector multislice CT exclude skeletal lesions during tumour staging? Implications for the cancer patient. Eur Radiol 16, 1066–1073 (2006). https://doi.org/10.1007/s00330-005-0042-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-005-0042-z