Abstract
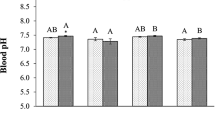
The effects of concurrent ocean warming and acidification on Antarctic marine benthos warrant investigation as little is known about potential synergies between these climate change stressors. We examined the interactive effects of warming and acidification on fertilization and embryonic development of the ecologically important sea urchin Sterechinus neumayeri reared from fertilization in elevated temperature (+1.5°C and 3°C) and decreased pH (−0.3 and −0.5 pH units) treatments. Fertilization using gametes from multiple males and females, to represent populations of spawners, was resilient to acidification at ambient temperature (0°C). At elevated temperatures, there was a negative interactive effect of temperature and pH on percentage of fertilization (11% reduction at 3°C). For cleavage stage embryos, there was a significant, but small reduction (6%) in the percentage of normal embryos at pH 7.5. For blastulae, a 10–11% decrease in normal development occurred in the +3°C treatments across all pH levels. Our results highlight the importance of considering the impacts of both temperature and pH in assessing the life history response of S. neumayeri in a changing polar ocean. While fertilization and development to the blastula stage were robust to levels of temperature and pH change predicted over coming decades, deleterious interactive effects were evident between these stressors at levels projected to occur by 2100 and beyond.



Similar content being viewed by others
References
Barnes DKA, Fuentes V, Clarke A, Schloss IR, Wallace MI (2006) Spatial and temporal variation in shallow seawater temperatures around Antarctica. Deep Sea Res II 53:853–865
Bay S, Burgess R, Nacci D (1993) Status and applications of echinoid (phylum Echinodermata) toxicity test methods. In: Landis WG, Hughes JS, Lewis MA (eds) Environmental toxicology and risk assessment. American Society for Testing and Materials, Philadelphia, pp 281–302
Benitez Villalobos F, Tyler PA, Young CM (2006) Temperature and pressure tolerances of embryos and larvae of the Atlantic seastars Asterias rubens and Marthasterias glacialis: potential for deep-sea invasion from the North Atlantic. Mar Ecol Prog Ser 314:109–117
Brewer PG (2009) A changing ocean seen with clarity. Proc Natl Acad Sci 106:12213–12214
Brey T, Pearse J, Basch L, McClintock JB, Slattery M (1995) Growth and production of Sterechinus neumayeri (Echinoidea: Echinodermata) in McMurdo Sound, Antarctica. Mar Biol 124:279–292
Byrne M (2010) Impact of climate change stressors on marine invertebrate life histories with a focus on the Mollusca and Echinodermata. In: Yu Y, Henderson-Sellers A (eds) Climate alert: climate change monitoring and strategy. Sydney, University of Sydney Press, pp 142–185
Byrne M (2011a) Global change ecotoxicology: identification of early life history bottlenecks in marine invertebrates, variable species responses and variable experimental approaches. Mar Env Res. doi:10.1016/j.marenvres.2011.10.004
Byrne M (2011b) Impact of ocean warming and ocean acidification on marine invertebrate life history stages: vulnerabilities and potential for persistence in a changing ocean. Oceanogr Mar Biol Ann Rev 49:1–42
Byrne M, Ho M, Selvakumaraswamy P, Nguyen HD, Dworjanyn SA, Davis AR (2009) Temperature, but not pH, compromises sea urchin fertilization and early development under near-future climate change scenarios. Proc R Soc B 276:1883–1935
Byrne M, Soars NA, Ho MA, Wong E, McElroy D, Selvakumaraswamy P, Dworjanyn SA, Davis AR (2010) Fertilization in a suite of coastal marine invertebrates from SE Australia is robust to near-future ocean warming and acidification. Mar Biol 157:2061–2069
Caldeira K, Wickett ME (2003) Anthropogenic carbon and ocean pH. Nature 425:365
Cao L, Caldeira K (2008) Atmospheric CO2 stabilization and ocean acidification. Geophys Res Lett. doi:10.1029/2008GL035072
Carr RS, Biedenbach JM, Nipper M (2006) Influence of potentially confounding factors on sea urchin porewater toxicity tests. Arch Env Cont Tox 51:573–579
Catarino, AI, Ridder, CD, Gonzalez M, Gallardo P, Dubois P (2011) Sea urchin Arbacia dufresnei (Blainville 1825) larvae response to ocean acidification. Polar Biol. doi:10.1007/s00300-011-1074-2
Christen R, Schackmann RW, Shapiro BM (1982) Elevation of the intracellular pH activates respiration and motility of sperm of the sea urchin, Strongylocentrotus purpuratus. J Biol Chem 257:4881–4890
Clark D, Lamare M, Barker M (2009) Response of sea urchin pluteus larvae (Echinodermata: Echinoidea) to reduced seawater pH: a comparison among tropical, temperate, and a polar species. Mar Biol 156:1125–1137
Clarke A, Murphy EJ, Meredith MP, King JC, Peck LS, Barnes DKA, Smith RC (2007) Climate change and the marine ecosystem of the western Antarctic Peninsula. Phil Trans R Soc B Biol Sci 362:149–166
Comeau S, Gorsky G, Jeffree R, Teyssie JL, Gattuso JP (2009) Impact of ocean acidification on a key Arctic pelagic mollusc (Limacina helicina). Biogeosci 6:1877–1882
Cowart DA, Ulrich PN, Miller DC, Marsh AG (2009) Salinity sensitivity of early embryos of the Antarctic sea urchin, Sterechinus neumayeri. Polar Biol 32:435–441
Darszon A, Guerrero A, Galindo BE, Nishigaki T, Wood CD (2008) Sperm-activating peptides in the regulation of ion fluxes, signal transduction and motility. Int J Devel Biol 52:595–606
Dickson AG, Sabine CL, Christian JR (2007) Guide to best practices for ocean CO2 measurements, vol. 3. PICES Special Publication, p 191
Doney SC, Fabry VJ, Feely RA, Kleypas JA (2009) Ocean acidification: the other CO2 problem. Ann Rev Mar Sci 1:169–192
Dupont S, Ortega-Martínez O, Thorndyke MC (2010) Impact of near future ocean acidification on echinoderms. Ecotoxicology 19:440–462
Ericson JA, Lamare MD, Morley SA, Barker MF (2010) The response of two ecologically important Antarctic invertebrates (Sterechinus neumayeri and Parborlasia corrugatus) to reduced seawater pH: effects on fertilisation and embryonic development. Mar Biol 157:2689–2702
Fabry VJ, McClintock JB, Mathis JT, Grebmeier JM (2009) Ocean acidification at high latitudes: the bellwether. Oceanography 22:160–171
Fujisawa H (1989) Differences in temperature dependence of early development of sea urchins with different growing seasons. Biol Bull 176:96–102
Gibson JE, Trull T (1999) Annual cycle of fCO2 under sea-ice and in open water in Prydz Bay, East Antarctica. Mar Chem 66:187–200
Hamdoun A, Epel D (2007) Embryo stability and vulnerability in an always changing world. Proc Natl Acad Sci USA 104:1745–1750
Hansen JE, Ruedy R, Glascoe J, Sato M (1999) GISS analysis of surface temperature change. J Geophys Res 104:30997–31022
Hoegh-Guldberg O, Bruno JF (2010) The impact of climate change on the world’s marine ecosystems. Science 328:1523–1528
Intergovernmental Panel on Climate Change (IPCC) (2007) Climate Change 2007: the fourth assessment report of the intergovernmental panel on climate change (IPCC). Cambridge University Press, Cambridge
King CK, Riddle MJ (2001) Effects of metal contaminants on the development of the common Antarctic sea urchin Sterechinus neumayeri and comparisons of sensitivity with tropical and temperate echinoids. Mar Ecol Prog Ser 215:143–154
Krug PJ, Rifell JA, Zimmer RK (2009) Endogenous signaling pathways and chemical communication between sperm and egg. J Exp Biol 212:1092–1100
Kupriyanova EK, Havenhand JN (2005) Effects of temperature on sperm swimming behaviour, respiration and fertilization success in the serpulid polychaete, Galeolaria caesepitosa (Annelida: Serpulidae). Invert Repr Dev 48:7–17
Kurihara H, Shirayama Y (2004) Effects of increased atmospheric CO2 on sea urchin early development. Mar Ecol Prog Ser 274:161–169
Lamare MD, Barker MF (1999) In situ estimates of larval development and mortality in the New Zealand sea urchin Evechinus chloroticus (Echinodermata: Echinoidea). Mar Ecol Prog Ser 180:197–211
Lamare MD, Barker MF, Lesser MP (2007) In situ rates of DNA damage and abnormal develoment in Antarctic and non-Antarctic sea urchin embryos. Aquat Biol 1:21–32
Lister KN, Lamare MD, Burritt DJ (2010) Sea ice protects embryos of the Antarctic sea urchin Sterechinus neumayeri from oxidative damage due to naturally enhanced levels of UV-B radiation. J Exp Biol 213:1967–1975
McClintock JB (1994) Trophic biology of Antarctic shallow-water echinoderms. Mar Ecol Prog Ser 111:191–202
McNeil BI, Matear R (2008) Southern Ocean acidification: a tipping point at 450-ppm atmospheric CO2. Proc Natl Acad Sci USA 105:18860–18864
Melzner F, Gutowska MA, Langenbuch M, Dupont S, Lucassen M, Thorndyke MC, Bleich M, Pörtner HO (2009) Physiological basis for high CO2 tolerance in marine ectothermic animals: pre-adaptation through lifestyle and ontogeny? Biogeosci 6:2313–2331
Meredith MP, King JC (2005) Rapid climage change in the ocean west of the Antarctic Peninsula during the second half of the 20th Century. Geophys Res Lett 32:L19604
Mita M, Hino A, Yasumasu I (1984) Effect of temperature on interaction between eggs and spermatozoa of sea urchin. Biol Bull 166:68–77
Palumbi SR (1999) All males are not created equal: fertility differences depend on gamete recognition polymorphisms in sea urchins. Proc Natl Acad Sci USA 96:12632–12637
Pearse JS, McClintock JB, Bosch I (1991) Reproduction of Antarctic Benthic marine invertebrates: tempos, modes, and timing. American Zool 31:65–80
Peck LS (2005) Prospects for survival in the Southern Ocean: vulnerability of benthic species to temperature change. Antarctic Sci 17:497–507
Peck LS, Webb KE, Bailey DM (2004) Extreme sensitivity of biological function to temperature in Antarctic marine species. Funct Ecol 18:625–630
Pierrot D, Lewis E, Wallace DWR (2006) MS excel program developed for CO2 system calculations. ORNL/CDIAC-105a. Oak Ridge, Tennessee: carbon dioxide information analysis center, Oak Ridge National Laboratory, U.S. Department of Energy
Rumrill SS (1990) Natural mortality of marine invertebrate larvae. Ophelia 32:163–198
Rupp JH (1973) Effects of temperature on fertilization and early cleavage of some tropical echinoderms, with emphasis on Echinometra mathaei. Mar Biol 23:183–189
Sewell MA, Young CM (1999) Temperature limits to fertilization and early development in the tropical sea urchin Echinometra lucunter. J Exp Mar Biol Ecol 236:291–305
Sheppard Brennand H, Soars N, Dworjanyn SA, Davis AR, Byrne M (2010) Impact of ocean warming and ocean acidification on larval development and calcification in the sea urchin Tripneustes gratilla. PlosOne 5:e11372
Sorte CJB, Jones SJ, Miller LP (2011) Geographic variation in temperature tolerance as an indicator of potential responses to climate change. J Exp Mar Biol Ecol 400:209–217
Stanwell-Smith D, Peck LS (1998) Temperature and embryonic development in relation to spawning and field occurrence of larvae of three Antarctic echinoderms. Biol Bull 194:44–52
Staver JM, Strathmann RR (2002) Evolution of fast development of planktonic embryos to early swimming. Biol Bull 203:58–69
Sunday JM, Crim RN, Harley CDG, Hart MW (2011) Quantifying rates of evolutionary adaptation in response to ocean acidification. PLoS ONE 6. doi:10.1371/journal.pone.0022881
US EPA (2002) Short-term methods for estimating the chronic toxicity of effluents and receiving waters to marine and estuarine organisms. Third Edition. United States Environmental Protection Agency
Wright DA, Kennedy VS, Roosenburg WH, Castagna M, Mihursky JA (1983) Temperature tolerance of embryos and larvae of five bivalve species under simulated power plant entrainment conditions: a synthesis. Mar Biol 77:271–278
Zeebe RE, Wolf-Gladrow D (2005) CO2 in seawater: equilibrium, kinetics, isotopes. Elsevier Oceanography Series 65. Elsevier Inc, San Diego
Acknowledgments
This work was supported by a grant from the Australian Antarctic Division (AAD). Thanks to AAD staff and students who provided assistance and logistical support including Rob King, Steve Whiteside, John van den Hoff, Andrew Bryant, Clive Strauss, Charmaine Alford, Kathryn Brown, Lara Marcus, Sarah Payne, Bianca Sfiligoj, Claire Wallis, Debbie Lang, Jane Wasley, Dougie Gray, and Leigh Hornsby.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ericson, J.A., Ho, M.A., Miskelly, A. et al. Combined effects of two ocean change stressors, warming and acidification, on fertilization and early development of the Antarctic echinoid Sterechinus neumayeri . Polar Biol 35, 1027–1034 (2012). https://doi.org/10.1007/s00300-011-1150-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-011-1150-7