Abstract
In genetic transformation systems, antibiotic resistance genes are routinely used as powerful markers for selecting transformed cells from surrounding non-transformed cells. However, simultaneous use of the gene encoding green fluorescent protein (GFP) and an antibiotic resistance gene facilitates the selection process, since it allows visible selection of transformed cells. Here, we report the development of a visual selection system for transformed cells using a GFP marker without selection against antibiotics after Agrobacterium-mediated transformation in rice. Both GFP protein levels and GFP fluorescence in calli isolated by visual selection were higher than in calli selected on hygromycin (Hyg), suggesting that transgenic calli hyper-accumulating GFP were efficiently obtained by selection using GFP fluorescence itself rather than Hyg resistance. Furthermore, gfp transcripts in calli isolated by visual selection were more abundant than under Hyg selection; in contrast, transcript levels of hpt in calli selected visually were comparable to those obtained under Hyg selection. These results suggest that there was no correlation between hpt and gfp expression levels, despite the fact that they are aligned in tandem on an integrated locus after selection by either GFP fluorescence or Hyg resistance. This fact indicates that positional effects can influence the expression of each transgene differently, even when they are located in tandem at the same locus. In summary, based on our results, we discuss a model system for rice cell culture transformation for the production of recombinant proteins using visual selection.
Similar content being viewed by others
Introduction
The ideal selection marker in genetic transformation should be readily expressed, easily visualized and non-toxic for humans and the environment (Hraska et al. 2006). Fluorescent proteins such as green fluorescent protein (GFP), whose discovery and development led to the award of the Nobel Prize in Chemistry in 2008, are the most useful visual markers (Shimomura et al. 1962). Fluorescent proteins enable direct observation and an easier and more precise evaluation of transformation events in real time under living conditions, without treatment with any exogenous substrate. Moreover, GFP fluorescence was shown to be an accurate tool for protein quantification and can be used as a quantitative selection marker and an indicator of gene expression (Niwa et al. 1999; Richards et al. 2003a; Stewart 2005). Visual selection using fluorescent proteins is a powerful tool in cases, where the callus proliferation or regeneration step is inefficient under antibiotic or herbicide selection, because it enables selection and regeneration of transgenic plant cells without any additional selection pressure (Hraska et al. 2006; Stewart 2001). In addition, the use of antibiotic resistance markers such as hygromycin phosphotransferase (hpt) has been a subject of public concern, due to the possible deleterious effects on other organisms in the ecosystem caused by the use of antibiotics. Thus, the development of new markers for risk-free genetic engineering for crop improvement is highly desirable. Visual selection could be one such strategy. To date, visual selection using GFP has been performed successfully in various plants (Hraska et al. 2006; Stewart 2001). However, to achieve visual selection without antibiotics, a highly efficient transformation system is required, as transformed cells have to be completely separated from the much larger fraction of untransformed cells (Hraska et al. 2006).
Since the first reliable report of rice transformation with Agrobacterium (Hiei et al. 1994), Agrobacterium-mediated transformation technology in rice has been used widely in the fields of both molecular breeding and functional genomics applications such as the generation of T-DNA insertion lines. Until now, routine and efficient Agrobacterium-mediated transformation systems have been developed for japonica, javanica and indica cultivars of Asian rice (Oryza sativa L.) and for the new rice for Africa (NERICA, O. sativa × O. glaberrima) (Dong et al. 1996; Hiei and Komari 2006; Hiei et al. 1994; Ishizaki and Kumashiro 2008; Rashid et al. 1996; Toki 1997; Toki et al. 2006). In Nipponbare, a model variety of japonica rice, the generation of transgenic plantlets at high frequency was successfully achieved within a month of inoculation of mature seeds (Toki et al. 2006). In this system, several hundreds of independent transformation events occur in each small piece of callus (5–10 mm diameter). Moreover, sGFP (S65T), which has a fluorescent signal up to 100-fold brighter than that of the original jellyfish GFP in plants, enables transformed and non-transformed cells in rice callus to be clearly distinguished (Niwa 2003; Niwa et al. 1999). Thus, the Agrobacterium-mediated transformation system in Nipponbare using this modified GFP seemed a good candidate for development of a visual selection system. Although visual selection using a GFP marker without antibiotic selection has previously been performed in a rice transformation system (particle bombardment of immature embryos; Vain et al. 1998), the application of visual selection to Agrobacterium-mediated transformed callus has not yet been reported.
In this study, we established Agrobacterium-mediated transformation of rice using GFP as a visible selection marker, and characterized transformed plants obtained via visual selection. Furthermore, careful comparison of the visual selection system using GFP and antibiotic selection using Hyg suggested that GFP was suitable not only as a selection marker, but also as an indicator of high gene expression levels during selection of transformed calli. Finally, we discuss the potential application of visual selection in rice cell culture for the production of recombinant proteins.
Materials and methods
Binary vector and Agrobacterium transformation
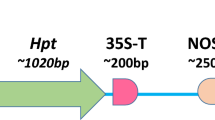
The binary plasmid vector pCAMBIA–sGFP (Fig. 1a) used in this study (Toki 1997; Toki et al. 2006) was transferred into Agrobacterium tumefaciens strain EHA105 by electroporation (Hood et al. 1993).
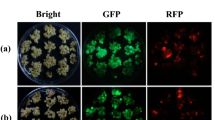
Schematic representation of visual selection in rice callus. a Structure of the T-DNA used in this study. The GFP and hpt expression cassettes were cloned in the binary vector pCAMBIA1390 (Niwa 2003; Niwa et al. 1999; Toki et al. 2006), yielding pCAMBIA–sGFP. GFP expression was under the control of the CaMV 35S promoter (35S pro) and a duplicated nopaline synthase terminator (2 × NOS ter); hpt expression was directed by a duplicated 35S promoter (2 × 35S pro) and the CaMV 3′UTR. The orange bar indicates the region covered by the DIG-labeled hpt probe used for Southern blot analysis. b Visual selection in rice callus. Rice calli were transformed with Agrobacterium harboring the vector shown in (a), and cultured without antibiotic selection. Calli including sectors emitting green fluorescence (as indicated by the red circle) were isolated under blue light and propagated. c Conventional antibiotic selection in rice callus. Transformed rice calli were cultured in the presence of pCAMBIA–sGFP hygromycin, and vigorously growing sectors (red circle) were isolated under white light and propagated
Plant materials
A japonica-type rice (Oryza sativa L. cv. Nipponbare) was used in this study. For extraction of DNA, RNA or protein, rice calli or leaves were harvested, immediately frozen in liquid N2 and stored at −80°C.
Visual selection procedure
Dehulled seeds, which were sterilized with 70% ethanol and 2.5% sodium hypochlorite, were inoculated on callus induction N6D medium solidified with 0.4% gelrite, and cultured for 7 days at 33°C (light for 10 h)/30°C (dark for 14 h). Selection against hygromycin (Hyg) and regeneration of transgenic plants were performed following the method of Toki et al. (2006). Visual selection of GFP was performed as follows: after 3-days of co-cultivation with Agrobacterium at 25°C under constant dark, the calli were washed and cultured on N6D medium containing 400 mg/L carbenicillin (Nakalai tesque, Kyoto, Japan) for 4–8 weeks at 33°C (light for 10 h)/30°C (dark for 14 h). Every 2 weeks, calli sectors emitting GFP fluorescence were transferred to fresh N6D medium. GFP fluorescence was observed using a fluorescence microscope with a GFP2 filter (MZ FLIII, Leica Microsystems, Wetzlar, Germany). Selected calli expressing GFP were regenerated on Re-III medium at 30°C under constant light. Shoots arising from calli on Re-III medium were transferred to HF medium to allow vigorous growth of roots.
PCR and Southern blot analysis
Genomic DNA was extracted from leaves using Nucleon PhytoPure (GE Healthcare, Piscataway, NJ, USA) according to the manufacturer’s protocol. PCR analysis was performed using KOD dash DNA polymerase (TOYOBO, Osaka, Japan) with primer sets 5′-ATAGCTGCGCCGATGGTT-3′/5′-CGTCTGCTGCTCCATACAAG-3′ for hpt, and 5′-TGTGATATCTTCCACTGACGTAAGGGA-3′/5′-TGTACTCCAGCTTGTGCCCCAGGAT-3′ for GFP. Southern blot analysis was performed according to a standard protocol. A specific DNA probe for the hpt gene was prepared using a PCR digoxigenin (DIG) probe synthesis kit (Roche Diagnostics, Basel, Switzerland) according to the manufacturer’s protocol with the primer set described above. Southern blot hybridization signals were detected and analyzed using a Lumivision PRO (TAITEC, Saitama, Japan).
Quantitative RT-PCR
Total RNA was extracted from frozen calli with an RNeasy Plant Mini Kit (Qiagen, Hilden, Germany). Transcript levels of each gene were measured by real-time quantitative RT-PCR using a Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) and an ABI7300 (Applied Biosystems) according to the manufacturers’ protocols. The gene-specific primers used for quantitative RT-PCR were 5′-CAGAAGAACGGCATCAAGGT-3′/5′-CTGGGTGCTCAGGTAGTGGT-3′ for gfp, and 5′-ATAGCTGCGCCGATGGTT-3′/5′-GGGAGATGCAATAGGTCAGG-3′ for hpt.
Immuno-blot analysis and measurement of GFP fluorescence
Total protein was extracted from frozen calli with extraction buffer (0.1 M sodium phosphate, 0.1% Triton-X and 20% glycerol). Electrophoresis of protein and immuno-blot hybridization were performed as described previously (Osakabe et al. 2006). Living colors A.v. (JL-8) monoclonal antibody (Clontech, Mountain View, CA, USA) was used as the antibody against GFP, and recombinant GFP protein (Clontech) was used as a standard for protein quantification. Signals were detected and analyzed as for Southern blot hybridization. A blot was stained by MemCode Reversible Protein Stain Kit (PIERCE, Illinois, USA) to visualize proteins and estimate protein loading. Quantification of GFP fluorescence was carried out using a Bio-Rad Molecular Imager FX in combination with Quantity One software (Bio-Rad).
Results
Establishment of a visual selection system for rice transformation
Visual selection using GFP fluorescence without antibiotics was performed on rice calli infected with Agrobacterium containing pCAMBIA–sGFP (Fig. 1a). After a 3-day co-cultivation with Agrobacterium, rice calli were washed to remove Agrobacterium and cultured on N6D medium containing carbenicillin. Two weeks after Agrobacterium infection, sectors emitting green fluorescence were isolated under a stereoscopic microscope and subcultured at 10- to 20-day intervals (Fig. 1b). Strong green fluorescence emitted from rice callus was considered to be derived from the rice cells because only a faint GFP signal was shown to be detected from Agrobacterium harboring pCAMBIA–sGFP (Toki et al. 2006). In parallel, antibiotic selection using Hyg was performed following the method of Toki (1997; Toki et al. 2006); 2 weeks after Agrobacterium infection, sectors growing vigorously in the presence of Hyg were isolated under white light and sub-cultured (Fig. 1c).
Using the visual selection system, we succeeded in achieving clonal propagation of transformed calli and regeneration of transformed plants. The efficiency of clonal propagation (the ratio of the number of clonal lines successfully propagated to the number of Agrobacterium-infected calli) obtained by visual selection was estimated at 10–20%, compared to the almost 90% achieved using Hyg selection (Table 1). The final transformation efficiency (the ratio of the number of regenerated plant lines in which T-DNA insertion was confirmed to that of Agrobacterium-infected callus) did not exceed 10%, compared to the >80% achieved in the conventional Hyg system (Table 1).
Characterization of transgenic plants selected using GFP fluorescence
Southern hybridization analysis was performed to evaluate the presence of the T-DNA in the T0 generation of transgenic rice plants. Transgenic plants selected with GFP contained single (Fig. 2a, lanes 3, 6, 9 and 10) or multiple (lanes 2, 4, 7 and 8) hybridizing bands. On average, 2.3 T-DNA copies were integrated in rice plants selected with GFP (n = 13; Fig. 2a, and data not shown). Previously, we have reported that, on average, 2.1 copies of T-DNA were integrated in rice plants selected on Hyg in our transformation system (Nakamura et al. 2007), suggesting that the integrated T-DNA copy number in calli isolated by visual selection is comparable to that in calli selected on Hyg.
Genetic analysis of transgenic plants obtained by visual selection. a Southern blot analysis using the hpt probe shown in Fig. 1a of HindIII-digested genomic DNA extracted from regenerated plants. Lanes: NT non-transformant, 1 plant from transgenic calli obtained by Hyg selection, 2–10 plants from transgenic calli obtained by visual selection. b Southern blot analysis as in (a) with plants from the T1 generation. Lanes: NT non-transformant, T 0 T0 plant, T 1 (1–8) plants of the T1 generation. c Immuno-blot analysis of GFP protein expression in plants of the T1 generation. Twenty microgram of total proteins extracted from young leaves were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Lanes: NT non-transformant, T 0 T0 plant, T 1 (1–8) plants of the T1 generation. d GFP fluorescence in T1 transgenic plants T1-3 and T1–7. Upper and lower panels show images taken under blue and white light, respectively
The stable inheritance of transgenes to the T1 generation of transformed plants was evaluated by Southern blot analysis and immuno-blot analysis. Southern blot analysis revealed the transmission and segregation of transgene in T1 plants (Fig. 2b). Similarly, analysis of GFP protein confirmed the transmission and segregation of transgene in T1 plants (Fig. 2c, d). Moreover, GFP fluorescence was observed in 42 of 62 T1 seeds, which fits a 3:1 ratio (χ2 = 1.74; P > 0.05). These results showed that, just as with selection on Hyg, transgenes in transformed plants selected visually using GFP fluorescence are stably inherited to the T1 generation in a Mendelian manner.
Gene expression analysis of transgenic calli selected with GFP fluorescence
Quantification by immunoblot analysis of GFP protein extracted from calli revealed concentrations of 13.7 and 16.1 ng/μg soluble total protein in calli isolated by visual selection (Fig. 3a, lanes 3, 4), while 1.4 and 1.6 ng/μg total soluble protein in calli were selected on Hyg (lanes 1, 2). In addition, GFP fluorescence emitted from protein extracted from calli was quantified. Just as with the GFP protein level, GFP fluorescence in calli isolated by visual selection was higher than that in calli isolated by Hyg selection (Fig. 3b). Furthermore, we cultured calli selected by GFP in N6D liquid medium (Fig. 3a, lane 4). After a 2-week cultivation, a large amount of GFP protein (estimated as 84 ng/μg total protein) had leaked into the liquid medium (Fig. 3c). These results suggested that GFP hyper-accumulating transgenic lines were efficiently obtained using our visual selection system.
Expression analysis in transgenic calli selected visually or on hygromycin. a Immuno-blot analysis of GFP protein expression in transformed calli. Total soluble proteins extracted from transformed calli selected on Hyg (lanes 1 and 2; 20 μg) or visually by GFP fluorescence (lanes 3 and 4; 2 μg) were separated by SDS-PAGE and analyzed with an anti-GFP antibody. Upper panel western blot analysis. Lower panel a membrane stained by MemCode Reversible Protein Stain Kit after blotting. b Comparison of GFP protein concentration with GFP fluorescence in total soluble protein extracted from transformed calli selected with GFP or Hyg. Black diamonds, grey squares and white circles indicate transformed calli selected with 50 mg/L Hyg (Hyg50), 100 mg/L Hyg (Hyg100) or GFP (0 mg/L Hyg, visual), respectively. c Two-week-old liquid medium culture of calli selected with GFP. The right and left images were taken under white and blue light, respectively. d mRNA levels of gfp and hpt genes in transformed calli. For qRT-PCR, 0.1 μg total RNA extracted from transformed calli was used as a template. All mRNA levels were normalized to the OsActin1 level as a control and are presented as the ratio relative to lane 1. Data are the mean ± SD of three separate PCR analyses. The gray and white bars indicate the mRNA levels of GFP and hpt, respectively. 1, 2 Transgenic calli obtained by Hyg selection 3, 4 transgenic calli obtained by visual selection
Thus, using GFP fluorescence as a visual selection indicator, we succeeded in the clonal propagation of calli in which GFP protein was accumulated to a high level (Fig. 3a, b), raising the possibility that calli expressing high amounts of GFP protein could be selected under high Hyg pressure, because the hpt and gfp expression cassettes are aligned in tandem and the hpt expression level was thus assumed to be correlated with that of gfp. To test this, transformed calli selected on 50 mg/L (usual concentration) or 100 mg/L (high concentration) Hyg were analyzed. With selection on 100 mg/L Hyg, the efficiency of clonal propagation (the ratio of the number of lines successfully propagated to the number of Agrobacterium-infected calli) was approximately 80%; efficiency was slightly lower on 50 mg/L Hyg (Table 2). Quantification of GFP protein and GFP fluorescence showed that no lines of calli in which GFP protein was accumulated to a high level were obtained with selection on Hyg (Fig. 3b), suggesting that it is difficult to obtain transformed calli accumulating high levels of GFP protein under Hyg selection pressure, even if higher Hyg pressure, at least 100 mg/L Hyg, is used.
The mRNA levels of gfp and hpt gene transcripts were analyzed by quantitative RT-PCR. The mRNA level of the gfp gene in calli isolated by visual selection was approximately fivefold higher than with Hyg selection (Fig. 3d). On the other hand, hpt gene mRNA levels in calli isolated by visual selection were comparable to those obtained following Hyg selection (Fig. 3d), suggesting that transcription of the hpt and gfp genes is not co-regulated.
Discussion
In this study, transgenic rice plants mediated by Agrobacterium were selected using GFP as a visible marker. Stable integration of T-DNA in the T0 generation and inheritance to the T1 generation were confirmed (Fig. 2). To date, visual selection in rice using a GFP marker without antibiotic selection has previously been performed through particle bombardment (Vain et al. 1998). Genetic transformation mediated by Agrobacterium is superior to particle bombardment method in some points. Agrobacterium-mediated transformation results in less rearrangement and lower copy insertion of transgenes. Moerover, transgenes tend to integrate into transcriptionally active regions. Thus, visual selection system using GFP mediated by Agrobacterium is thought to be more useful and practically applicable compared to particle bombardment.
The transformation efficiency with visual selection was lower than that with conventional Hyg selection because of the technical difficulty of clonal propagation of transformed calli selected on the basis of GFP fluorescence (Table 1). Other authors who have used visual selection of rice, sugarcane and wheat transformants after bombardment also found it very difficult to maintain preferential growth of transformed cells (Elliott et al. 1999; Jordan 2000; Vain et al. 1998). At least two factors are thought to be necessary to enhance the efficiency of clonal propagation in visual selection systems in rice: (1) an increase in the number of transformed cells, in which integrated genes are stably expressed, and (2) vigorous growth of transformed cells versus non-transformed cells. We are currently analyzing these factors to improve the efficiency of our visual selection system.
By using GFP visual selection system, we succeeded in the clonal propagation of calli in which GFP mRNA and protein accumulated to high levels (Fig. 3). However, unlike in lines selected on Hyg, hpt and gfp gene transcript levels were not correlated in calli lines isolated by visual selection (Fig. 3d). In addition, selection with a higher concentration of Hyg did not enhance accumulation of GFP protein (Fig. 3b). These results suggest that selection relies on the appropriate level of marker gene expression; selection pressure in GFP selection is thought to be dependent not on hpt expression, but on GFP expression, and positional effects on the transcription of each transgene differ even when they are located in tandem at the same locus. A similar result was reported in Arabidopsis (Hsiao et al. 2007). Hsiao et al. developed a new selection system in Arabidopsis based on the overexpression of the Arabidopsis tryptophan synthase beta 1 (AtTSB1) gene and selection with 5-methyl-tryptophan (5MT) or CdCl2. Selection of transformed Arabidopsis with 5MT or CdCl2 showed that TSB1 mRNA levels increased despite uneven expression level of the hpt gene (Hsiao et al. 2007). These results support the notion that the expression level of the selection gene is determined by the selection method. For the production of recombinant proteins, visual selection appears to be useful method in spite of the low efficiency of clonal propagation.
Plant cell culture systems are regarded as a favorable means of producing small-to-medium quantities of high-priced, high-purity and specialty recombinant proteins. Recombinant proteins produced by transformed plant cells are more likely to be safe for human consumption, because there is no risk of contamination not only by agrochemicals, but also by human pathogens and endotoxins (Hellwig et al. 2004). Furthermore, GFP was found to be non-toxic for rats when ingested either in a purified form or expressed in transgenic plants (Richards et al. 2003b), suggesting that the combination of a plant cell culture system with a visual selection system based on GFP would be acceptable. In this study, we obtained calli in which GFP protein accumulated to about 15 ng/μg total soluble proteins (Fig. 3a, b). In the liquid culture in this callus line, 84 ng GFP was secreted per 1 μg total protein in 2 μL cultured medium (see Fig. 3c). In previous reports, recombinant proteins such as antibodies or lysozyme accumulated to ~3–4% of total soluble proteins and 85–200 mg/L in liquid culture medium (Hellwig et al. 2004). GFP accumulation in calli selected visually was less than half that in previous reports; this is most likely due to the difference in rice cultivar or promoter used to drive target gene expression. Stability of transgene products in plant cells also affects the level of exogenous protein accumulation.
In summary, we propose a model system of rice cell culture for the production of recombinant proteins using visual selection. Stewart (2005) discussed that a protein fusion would, a priori, be a better method of using GFP to monitor the accumulation of a protein of interest. Indeed, we demonstrated here that expression of the hpt gene did not correlate with that of the gfp gene, although they are tandemly aligned (Fig. 3d). This result suggests that a target gene should be fused to the gfp gene in order to be positively correlated with the expression of both genes. To achieve separate expression of the target protein, the inclusion of an internal ribosome entry sites (IRES) that would allow cap-independent translation initiation, or 2A oligopeptides that mediate self-cleavage at the C-terminus that could allow efficient separation of GFP and the recombinant protein, are strategies that could be considered (de Felipe et al. 2006; Matsuo et al. 2004; Stewart 2005; Urwin et al. 2000). There is a positive association between estimated GFP protein concentration and GFP fluorescence (R 2 = 0.82, Fig. 3b) as shown in a previous report (Richards et al. 2003a). In the selection step, calli emitting the strongest GFP fluorescence are propagated and cultured in liquid medium because the GFP-target fusion gene is assumed to be expressed at high level in such calli. In this study, abundant GFP protein leaked into the medium in liquid culture (Fig. 3c), suggesting the possibility that visible selection using GFP as an indicator of gene expression could be applied to the production of valuable recombinant proteins by liquid culture. In liquid culture of high GFP-fluorescent callus, a target recombinant protein of less than 30 kDa would be secreted and accumulate in the culture medium (Hellwig et al. 2004).
In conclusion, a visual selection system using GFP fluorescence can be successfully applied to Agrobacterium-mediated transformation in rice. Analysis of the expression levels of the gfp and hpt genes in the calli selected visually or on Hyg showed that the expression levels of these genes were not correlated, and that visual selection allows selection of calli in which the gfp gene is expressed to a high level.
Abbreviations
- GFP:
-
Green fluorescent protein
- Hyg:
-
Hygromycin
- hpt:
-
Hygromycin phosphotransferase
References
de Felipe P, Luke GA, Hughes LE, Gani D, Halpin C, Ryan MD (2006) E unum pluribus: multiple proteins from a self-processing polyprotein. Trends Biotechnol 24:68–75
Dong JJ, Teng WM, Buchholz WG, Hall TC (1996) Agrobacterium-mediated transformation of Javanica rice. Mol Breed 2:267–276
Elliott AR, Campbell JA, Dugdale B, Brettell RIS, Grof CPL (1999) Green fluorescent protein facilitates rapid in vivo detection of genetically transformed plant cells. Plant Cell Rep 18:707–714
Hellwig S, Drossard J, Twyman RM, Fischer R (2004) Plant cell cultures for the production of recombinant proteins. Nat Biotechnol 22:1415–1422
Hiei Y, Komari T (2006) Improved protocols for transformation of indica rice mediated by Agrobacterium tumefaciens. Plant Cell Tissue Organ Cult 85:271–283
Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza-sativa L) mediated by Agrobacterium and sequence-analysis of the boundaries of the T-DNA. Plant J 6:271–282
Hood EE, Gelvin SB, Melchers LS, Hoekema A (1993) New Agrobacterium helper plasmids for gene-transfer to plants. Transgenic Res 2:208–218
Hraska M, Rakousky S, Curn V (2006) Green fluorescent protein as a vital marker for non-destructive detection of transformation events in transgenic plants. Plant Cell Tissue Organ Cult 86:303–318
Hsiao P, Sanjaya SuRC, da Silva JAT, Chan MT (2007) Plant native tryptophan synthase beta 1 gene is a non-antibiotic selection marker for plant transformation. Planta 225:897–906
Ishizaki T, Kumashiro T (2008) Genetic transformation of NERICA, interspecific hybrid rice between Oryza glaberrima and O-sativa, mediated by Agrobacterium tumefaciens. Plant Cell Rep 27:319–327
Jordan MC (2000) Green fluorescent protein as a visual marker for wheat transformation. Plant Cell Rep 19:1069–1075
Matsuo N, Gilmartin PM, Hiratsuka K (2004) Characterization of the EMCV-IRES mediated bicistronic translation in plant cells. Plant Biotechnol 21:119–126
Nakamura H, Hakata M, Amano K, Miyao A, Toki N, Kajikawa M, Pang J, Higashi N, Ando S, Toki S, Fujita M, Enju A, Seki M, Nakazawa M, Ichikawa T, Shinozaki K, Matsui M, Nagamura Y, Hirochika H, Ichikawa H (2007) A genome-wide gain-of-function analysis of rice genes using the FOX-hunting system. Plant Mol Biol 65:357–371
Niwa Y (2003) A synthetic Green Fluorescent Protein gene for plant biotechnology. Plant Biotechnol 20:1–11
Niwa Y, Hirano T, Yoshimoto K, Shimizu M, Kobayashi H (1999) Non-invasive quantitative detection and applications of non-toxic, S65T-type green fluorescent protein in living plants. Plant J 18:455–463
Osakabe K, Abe K, Yoshioka T, Osakabe Y, Todoriki S, Ichikawa H, Hohn B, Toki S (2006) Isolation and characterization of the RAD54 gene from Arabidopsis thaliana. Plant J 48:827–842
Rashid H, Yokoi S, Toriyama K, Hinata K (1996) Transgenic plant production mediated by Agrobacterium in Indica rice. Plant Cell Rep 15:727–730
Richards HA, Halfhill MD, Millwood RJ, Stewart CN (2003a) Quantitative GFP fluorescence as an indicator of recombinant protein synthesis in transgenic plants. Plant Cell Rep 22:117–121
Richards HA, Han CT, Hopkins RG, Failla ML, Ward WW, Stewart CN (2003b) Safety assessment of recombinant green fluorescent protein orally administered to weaned rats. J Nutr 133:1909–1912
Shimomura O, Johnson FH, Saiga Y (1962) Extraction, purification and properties of aequorin, a bioluminescent protein from Luminous hydromedusan, Aequorea. J Cell Comp Physiol 59:223–240
Stewart CN (2001) The utility of green fluorescent protein in transgenic plants. Plant Cell Rep 20:376–382
Stewart CN (2005) Monitoring the presence and expression of transgenes in living plants. Trends Plant Sci 10:390–396
Toki S (1997) Rapid and efficient Agrobacterium-mediated transformation in rice. Plant Mol Biol Rep 15:16–21
Toki S, Hara N, Ono K, Onodera H, Tagiri A, Oka S, Tanaka H (2006) Early infection of scutellum tissue with Agrobacterium allows high-speed transformation of rice. Plant J 47:969–976
Urwin P, Yi L, Martin H, Atkinson H, Gilmartin PM (2000) Functional characterization of the EMCV-IRES in plants. Plant J 24:583–589
Vain P, Worland B, Kohli A, Snape JW, Christou P (1998) The green fluorescent protein (GFP) as a vital screenable marker in rice transformation. Theor Appl Genet 96:164–169
Acknowledgments
We thank Y. Niwa for providing sGFP and A. Baba for providing pCAMBIA1390–sGFP. We also thank K. Osakabe, K. Abe, M. Endo, S. Nonaka and N. Ohtsuki for stimulating discussion, and K. Amagai, R. Aoto, C. Furusawa, A. Nagashii, E. Ozawa and F. Suzuki for technical help. This work was supported financially by a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan to H.S. and S.T., and grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan to H.S. This work was also supported by a Program for Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN) grant to S.T. and the Budget for Nuclear Research of the Ministry of Education, Culture, Sports, Science and Technology, based on screening and counseling by the Atomic Energy Commission.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Toriyama.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Saika, H., Toki, S. Visual selection allows immediate identification of transgenic rice calli efficiently accumulating transgene products. Plant Cell Rep 28, 619–626 (2009). https://doi.org/10.1007/s00299-009-0671-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-009-0671-9