Abstract
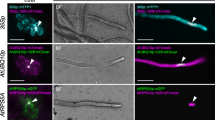
Effective containment of gene flow in transgenic plants requires a promoter that is highly specific for male and female gametes or tissues. Here, we report the creation of a novel pollen-, stigma- and carpel-specific (PSC) promoter through the fusion of the pollen-specific LAT52 and carpel-specific AGL5 enhancers to a stigma-specific SLG promoter. Gene expression analysis showed that fusion of the LAT52 enhancer to the SLG promoter enables the latter to gain pollen-specific activity while the acquirement of carpel-specific activity requires the correct orientation of the inserted AGL5 enhancer in the PSC promoter, and only a forward- but not a reverse-oriented one is functional. The resulting fPSC promoter, when fused to DT-A, generated at least three aberrant gynoecium phenotypes. Type I plants exhibited shortened stigmatic tissues, resembling plants containing the DT-A gene controlled by the SLG promoter. However, type II and III plants displayed partial or complete ablation of gynoecia, and were unable to support the reproductive process. Type II and III plants also produced severely perturbed anthers and pollen in comparison to type I or SLG::DT-A plants, and transgenic pollen grains were unable, when out-crossed with control plants, to pass the transgene to the next generation in all plants examined, indicating that they are selectively eliminated. This tissue-specific ablation or perturbation is highly specific, and does not compromise vegetative growth. Evidently, the fPSC promoter faithfully acquires tissue specificity from the incorporated enhancers and promoter, and should have a practical application for transgene containment in non-fruit and -grain producing plant crops.





Similar content being viewed by others
References
Al-Ahmad H, Galili S, Gressel J (2004) Tandem constructs to mitigate transgene persistence: tobacco as a model. Mol Ecol 13:697–710
Avni A, Edelman M (1991) Direct selection for paternal inheritance of chloroplasts in sexual progeny of Nicotiana. Mol Gen Genet 225:273–277
Bate N, Twell D (1998) Functional architecture of a late pollen promoter: pollen-specific transcription is developmentally regulated by multiple stage-specific and co-dependent activator elements. Plant Mol Biol 37:859–869
Beals TP, Goldburg RB (1997) A novel cell ablation strategy blocks tobacco anther dehiscence. Plant Cell 9:1527–1545
Bevan M (1984) Binary Agrobacterium vectors for plant transformation. Nucl Acids Res 12:8711–8721
Block M, Debrouwer D (1993) Engineered fertility control in transgenic Brassica napus L: histochemical analysis of anther development. Planta 189:218–225
Block M, Debrouwer D, Moens T (1997) The development of a nuclear male sterility system in wheat: expression of the barnase gene under the control of tapetum specific promoters. Theor Appl Genet 95:125–131
Burgess DG, Ralston EJ, Hanson WG, Heckert M, Ho M, Jeng T, Palys JM, Tang K, Gutterson N (2002) A novel, two-component system for cell lethality and its use in engineering nuclear male-sterility in plants. Plant J 31:113–125
Clough SJ, Bent AF (1998) Floral Dip: a simple method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Collins C, Azmi P, Berru M, Zhu X, Shulman MJ (2006) A weakened transcriptional enhancer yields variegated gene expression. PLoS One 1:e33
Daniell H (2002) Molecular strategies for gene containment in transgenic crops. Nat Biotechnol 20:581–586
Daniell H, Datta R, Varma S, Gray S, Lee SB (1998) Containment of herbicide resistance through genetic engineering of the chloroplast genome. Nat Biotechnol 16:345–348
Dwyer KG, Kandasamy MK, Mahosky DI, Acciai J, Kudish BI, Miller JE, Nasrallah ME, Nasrallah JB (1994) A superfamily of S locus-related sequences in Arabidopsis: diverse structures and expression patterns. Plant Cell 6:1829–1843
Ellstrand NC (2001) When transgenes wander, should we worry? Plant Physiol 125:1543–1545
Ellstrand NC, Prentice HC, Hancock JF (1999) Gene flow and introgression from domesticated plants into their wild relatives. Annu Rev Ecol Syst 24:217–242
Flanagan CA, Hu Y, Ma H (1996) Specific expression of the AGL1 MADS-box gene suggests regulatory functions in Arabidopsis gynoecium and ovule development. Plant J 10:343–353
Gasser CS, Budelier KA, Smith AG, Shah DM, Fraley RT (1989) Isolation of tissue-specific cDNA from tomato pistils. Plant Cell 1:15–24
Gleba Y, Marillonnet S, Klimyuk V (2004) Design of safe and biologically contained transgenic plants: tools and technologies for controlled transgene flow and expression. Biotechnol Genet Eng Rev 21:325–367
Gressel J (1999) Tandem constructs: preventing the rise of superweeds. Trends Biotechnol 17:361–366
Hall L, Topinka K, Huffman J, Davis L, Allen A (2000) Pollen flow between herbicide-resistant Brassica napus is the cause of multiple-resistant B. napus volunteers. Weed Sci 48:688–694
Hartley RW (1988) Barnase and Barstar: expression of its cloned inhibitor permits expression of a cloned ribonuclease. J Mol Biol 202:913–915
Jefferson R (1988) Plant reporter genes: the GUS gene fusion system. In: Setlow JK, Hollaender A (eds) Genetic engineering: principles and methods. Plenum Press, New York, pp 247–263
Kandasamy MK, Thorsness MK, Rundle SJ, Goldberg ML, Nasrallah JB, Nasrallah ME (1993) Ablation of papillar cell function in Brassica flowers results in the loss of stigma receptivity to pollination. Plant Cell 5:263–275
Keenan RJ, Stemmer WPC (2002) Nontransgenic crops from transgenic plants. Nat Biotechnol 20:215–216
Khan MS, Maliga P (1999) Fluorescent antibioyic resistance marker for tracking plastid transformation in higher plants. Nat Biotechnol 17:910–915
Kobayashi K, Munemura I, Hinata K, Yamamura S (2006) Bisexual sterility conferred by the differential expression of Barnase and Barstar: a simple and efficient method of transgene containment. Plant Cell Rep 25:1347–1354
Kumar S, Dhingra A, Daniell H (2004a) Plastid-expressed betaine aldehyde dehydrogenase gene in carrot cultured cells, roots, and leaves confers enhanced salt tolerance. Plant Physiol 136:2843–2854
Kumar S, Dhingra A, Daniell H (2004b) Stable transformation of the cotton plastid genome and maternal inheritance of transgenes. Plant Mol Biol 56:203–216
Kuvshinov V, Koivu K, Kanerva A, Pehu E (2001) Molecular control of transgene escape from genetically modified plants. Plant Sci 160:517–522
Lännenpää M, Hassinen M, Ranki A, Holtta-Vuori M, Lemmetyinen J, Keinonen K, Sopanen T (2005a) Prevention of flower development in birth and other plants using a BpFULL1::BARNASE construct. Plant Cell Rep 24:69–78
Lännenpää M, Parkkinen S, Järvinen P, Lemmetyinen J, Vepsäläinen S, Savola T, Keinonen K, Keinänen M, Sopanen T (2005b) The expression and promoter specificity of the birch homologs for PISTILLATA/GLOBOSA and APETALA3/DEFICIENS. Physiol Plant 125:268–280
Lee H-S, Huang S, Kao T-H (1994) S proteins control rejection of incompatible pollen in Petunia inflata. Nature 367:560–563
Lemmetyinen J, Keinonen K, Sopanen T (2004) Prevention of the flowering of a tree, silver birch. Mol Breed 13:243–249
Lemmetyinen J, Pennanen T, Lännenpää M, Sopanen T (2001) Prevention of flower formation in dicotyledons. Mol Breed 7:341–350
Li Y, Chang Z, Smith WA, Ellis DR, Chen Y, Zheng X, Pei Y, Luo K, Zhao D, Yao Q, Duan H, Li Q (2004) Invasive ornamental plants: problems, challenges, and molecular tools to neutralize their invasiveness. Crit Rev Plant Sci 23:381–389
Liu Z, Franks RG, Klink VP (2000) Regulation of marginal tissue formation by LEUNIG and AINTEGUMENTA. Plant Cell 12:1879–1892
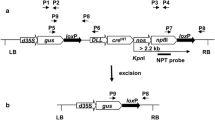
Luo K, Duan H, Zhao D, Zheng X, Deng W, Chen Y, Steward CN, McAvoy R, Jiang X, Wu Y, He A, Pei Y, Li Y (2007) “GM-gene-deletor”: fused LoxP-FRT recognition sequences dramatically improve the efficiency of FLP or Cre recombinase on transgene excision from pollen and seed of tobacco plants. Plant Biotechnol J 5:263–274
Mariani C, DeBeuckeleer M, Trueltner J, Leemans J, Goldberg RB (1990) Induction of male sterility in plants by a chimeric ribonuclease gene. Nature 347:737–741
Mariani C, Gossele V, Beuckeleer MD, Block MD, Goldburg RB, Greef WD, Leemans J (1992) A chimaeric ribonuclease-inhibitor gene restores fertility to male sterile plants. Nature 357:384–387
Mikkelsen TR, Andersen B, Jorgensen RB (1996) The risk of crop transgene spread. Nature 380:31–31
Nasrallah JB, Nasrallah ME (1993) Pollen-stigma signaling in the sporophytic self-incompatibility response. Plant Cell 5:1325–1335
Nasrallah JB, Yu S-M, Nasrallah ME (1988) Self-imcompatibility genes of Brassica oleracea: expression, isolation and structure. Proc Natl Acad Sci 85:5551–5555
Nilsson O, Wu E, Wolfe DS, Weigel D (1998) Genetic ablation of flowers in transgenic Arabidopsis. Plant J 15:799–804
Palmiter RD, Behringer RR, Quaife CJ, Maxwell FM, Maxwell IH, Brinster RL (1987) Cell lineage ablation in transgenic mice by cell-specific expression of a toxin gene. Cell 50:435–443
Roeder AHK, Ferrandize C, Yanofsky MF (2003) The role of the REPLUMLESS homeodomain protein in pattering the Arabidopsis fruit. Curr Biol 13:1630–1635
Ruf S, Hermann M, Berger IJ, Carrer H, Bock R (2001) Stable genetic transformation of tomato plastids—high-level foreign protein expression in fruit. Nat Biotechnol 19:870–875
Savidge B, Rounsley SD, Yanofsky MF (1995) Temporal relationship between the transcription of two Arabidopsis MADS box genes and the floral organ identity genes. Plant Cell 7:721–733
Skinner JS, Meilan R, Ma C, Strauss SH (2003) The Pupulus PTD promoter imparts floral-predominant expression and enables high levels of floral-organ ablation in Pupulus, Nicotiana and Arabidopsis. Mol Breed 12:119–132
Snow AA (2002) Transgenic crops—why gene flow matters. Nat Biotechnol 20:542–542
Snow AA, Palma PM (1997) Commercialization of transgenic plants: potential ecological risks. Bioscience 47:86–96
Thorsness MK, Kandasamy MK, Nasrallah ME, Nasrallah JB (1993) Genetic ablation of floral cells in Arabidopsis. Plant Cell 5:253–261
Twell D (1995) Diphtheria toxin-mediated cell ablation in developing pollen: vegetative cell ablation blocks generative cell migration. Protoplasma 187:144–154
Wei H, Meilan R, Brunner AM, Skinner JS, Ma K, Gandhi HT, Strauss SH (2007) Field trial detects incomplete barstar attenuation of vegetative cytotoxicity in Populus trees containing a poplar LEAFY promoter::barnase sterility transgene. Mol Breed 19:69–85
Weigel D, Ahn JH, Blazquez MA, Borevitz JO, Christensen SK, Fankhauser C, Ferrandiz C, Kardailsky I, Malancharuvil EJ, Neff MM, Nguyen JT, Sato S, Wang Z-Y, Xia J, Dixon RA, Harrison MJ, Lamb CJ, Yanofsky MF, Chory J (2000) Activation tagging in Arabidopsis. Plant Physiol 122:1003–1013
Acknowledgments
We thank Dennis Bennett and Lin Zhang for the help with generating transgenic plants, Dr. June Nasrallah for providing SLG13 promoter and Dr. Stacy Singer for critical reading of the manuscript. This work was supported by a grant from US Department of Agriculture (2006-03701) to ZL.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by R. Schmidt.
Rights and permissions
About this article
Cite this article
Liu, Z., Zhou, C. & Wu, K. Creation and analysis of a novel chimeric promoter for the complete containment of pollen- and seed-mediated gene flow. Plant Cell Rep 27, 995–1004 (2008). https://doi.org/10.1007/s00299-008-0522-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-008-0522-0