Abstract
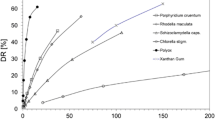
Xanthan gum is an important high polymer for turbulent drag reduction in fluid engineering applications, but the drag reduction characteristics will disappear with ultrahigh rotating shear. To further reveal this phenomenon, the average shear rate is proposed with the energy dissipation theory to explore drag reduction and rheological characteristics of the polymers in an ultrahigh rotating shear experimental system. The results indicate that the shear leads to the mechanical degradation of the polymer, further increasing the ultrahigh shear rate. A higher shear rate accelerates the degradation of the polymer solution and makes it approach Newtonian fluid, decreasing drag reduction characteristics. Besides, it is found that the ideal addition concentration is 3500 ppm, at which the relative degradation degree is globally slightest, and the relative degradation rate is locally smallest. Understanding these characteristics could help select the ideal concentration of polymers and add polymers regularly to maintain the drag reduction efficiency during ultrahigh rotating shear.











Similar content being viewed by others
References
Pereira AS, Soares EJ (2012) Polymer degradation of dilute solutions in turbulent drag reducing flows in a cylindrical double gap rheometer device. J Non-Newton Fluid 179–180:9–22. https://doi.org/10.1016/j.jnnfm.2012.05.001
Shetty AM, Solomon MJ (2009) Aggregation in dilute solutions of high molar mass poly (ethylene) oxide and its effect on polymer turbulent drag reduction. Polymer 50:261–270. https://doi.org/10.1016/j.polymer.2008.10.026
Tamano S, Ikarashi H, Morinishi Y, Taga K (2015) Drag reduction and degradation of nonionic surfactant solutions with organic acid in turbulent pipe flow. J Non-Newton Fluid 215:1–7. https://doi.org/10.1016/j.jnnfm.2014.10.011
Moosaie A, Manhart M (2011) An algebraic closure for the DNS of fiber-induced turbulent drag reduction in a channel flow. J Non-Newton Fluid 166:1190–1197. https://doi.org/10.1016/j.jnnfm.2011.07.006
Sokhal KS, Gangacharyulu D, Bulasara VK (2019) An experimental investigation of heterogeneous injection of biopolymer (guar gum) on the flow patterns and drag reduction percentage for two phase (water-oil mixture) flow. Expe Therm Fluid Sci 102:342–350. https://doi.org/10.1016/j.expthermflusci.2018.12.012
Pribush A, Hatzkelzon L, Meyerstein D, Meyerstein N (2013) The mechanism of the polymer-induced drag reduction in blood. Colloid Surface B 103:354–359. https://doi.org/10.1016/j.colsurfb.2012.11.004
Chen X, Su Y, Reay D, Riffat S (2016) Recent research developments in polymer heat exchangers–A review. Renew Sust Energ Rev 60:1367–1386. https://doi.org/10.1016/j.rser.2016.03.024
Bessa KL, Belletati JF, Dos Santos L, Rossoni LV, Ortiz JP (2011) Drag reduction by polyethylene glycol in the tail arterial bed of normotensive and hypertensive rats. Braz J Med Biol Res 44:767–777. https://doi.org/10.1590/s0100-879x2011007500071
Wrobel M (2020) On the application of simplified rheological models of fluid in the hydraulic fracture problems. Int J Eng Sci 150:103275. https://doi.org/10.1016/j.ijengsci.2020.103275
Kameneva MV (2012) Microrheological effects of drag-reducing polymers in vitro and in vivo. Int J Eng Sci 59:168–183. https://doi.org/10.1016/j.ijengsci.2012.03.014
Mehrabadi MA, Sadeghy K (2008) Simulating drag reduction phenomenon in turbulent pipe flows. Mech Res Commun 35:609–613. https://doi.org/10.1016/j.mechrescom.2008.06.003
Rasti E, Talebi F, Mazaheri K (2019) Improvement of drag reduction prediction in viscoelastic pipe flows using proper low-Reynolds k-ε turbulence models. Physica A 516:412–422. https://doi.org/10.1016/j.physa.2018.10.009
Pereira AS, Mompean G, Soares EJ (2018) Modeling and numerical simulations of polymer degradation in a drag reducing plane Couette flow. J Non-Newton Fluid 256:1–7. https://doi.org/10.1016/j.jnnfm.2018.03.007
Zhang Y, Zhou F, Li J, Kang J, Zhang Q (2019) Application and research of new energy-efficiency technology for liquid ring vacuum pump based on turbulent drag reduction theory. Vacuum 172:109076. https://doi.org/10.1016/j.vacuum.2019.109076
Makarim A, Seyede RY, Layth S, Masoud SN (2022) Green synthesis of DyBa2Fe3O7.988/ DyFeO3 nanocomposites using almond extract with dual eco-friendly applications: Photocatalytic and antibacterial activities. Int J Hydrogen Energ 31:14319–14330. https://doi.org/10.1016/j.ijhydene.2022.02.175
Yousefi SR, Ghanbari M, Amiri O, Marzhoseyni Z, Salavati-Niasari M (2021) Dy2BaCuO5/Ba4DyCu3O9.09 S cheme heterojunction nanocomposite with enhanced photocatalytic and antibacterial activities. J Am Ceram Soc 104(7):2952–2965. https://doi.org/10.1111/jace.17696
Yousefi SR, Alshamsi HA, Amiri O, Salavati-Niasari M (2021) Synthesis, characterization and application of Co/Co3O4 nanocomposites as an effective photocatalyst for discoloration of organic dye contaminants in wastewater and antibacterial properties. J Mol Liq 337:116405. https://doi.org/10.1016/j.molliq.2021.116405
Yousefi SR, Sobhani A, Alshamsi HA, Salavati-Niasari M (2021) Green sonochemical synthesis of BaDy2NiO5/Dy2O3 and BaDy2NiO5/NiO nanocomposites in the presence of core almond as a capping agent and their application as photocatalysts for the removal of organic dyes in water. RSC Adv 11:11500–11512. https://doi.org/10.1039/D0RA10288A
Yousefi SR, Amiri O, Salavati-Niasari M (2019) Control sonochemical parameter to prepare pure Zn0.35Fe2.65O4 nanostructures and study their photocatalytic activity. Ultrason Sonochem 58:104619. https://doi.org/10.1016/j.ultsonch.2019.104619
Yousefi SR, Masjedi-Arani M, Morassaei MS, Salavati-Niasari M (2019) Hydrothermal synthesis of DyMn2O5/Ba3Mn2O8 nanocomposite as a potential hydrogen storage material. Int J Hydrogen Energ 44:24005–24016. https://doi.org/10.1016/j.ijhydene.2019.07.113
Yousefi SR, Sobhani A, Salavati-Niasari M (2017) A new nanocomposite superionic system (CdHgI4/HgI2): synthesis, characterization and experimental investigation. Adv Powder Technol 28:1258–1262. https://doi.org/10.1016/j.apt.2017.02.013
Yousefi SR, Ghanbari D, Salavati-Niasari M, Hassanpour M (2016) Photo-degradation of organic dyes: simple chemical synthesis of Ni (OH)2 nanoparticles, Ni/Ni (OH)2 and Ni/NiO magnetic nanocomposites. J Mater Sci-Mater El 27:1244–1253. https://doi.org/10.1007/s10854-015-3882-6
Yousefi SR, Ghanbari D, Salavati-Niasari M (2016) Hydrothermal synthesis of nickel hydroxide nanostructures and flame retardant poly vinyl alcohol and cellulose acetate nanocomposites. J Nanostruct 6:77–82. https://doi.org/10.7508/jns.2016.01.013
Lim GH, Choi HJ, Renou F, Roy AN (2017) Effects of hydrophobic modification of xanthan gum on its turbulent drag reduction characteristics. J Ind Eng Chem 54:146–150. https://doi.org/10.1016/j.jiec.2017.05.027
Thais L, Gatski TB, Mompean G (2013) Analysis of polymer drag reduction mechanisms from energy budgets. Int J Heat Fluid Fl 43:52–61. https://doi.org/10.1016/j.ijheatfluidflow.2013.05.016
Lindner A, Vermant J, Bonn D (2003) How to obtain the elongational viscosity of dilute polymer solutions. Physica A 319:125–133. https://doi.org/10.1016/s0378-4371(02)01452-8
Escudier MP, Presti F, Smith S (1998) Drag reduction in the turbulent pipe flow of polymers. J Non-Newton Fluid 81:197–213. https://doi.org/10.1016/s0377-0257(98)00098-6
Ramadan A, Saasen A, Skalle P (2004) Application of the minimum transport velocity model for drag-reducing polymers. J Petrol Sci Eng 44:303–316. https://doi.org/10.1016/j.petrol.2004.03.002
Metzner AB, Otto RE (2010) Agitation of non-newtonian fluids. AICHE J 3:3–10. https://doi.org/10.1002/aic.690030103
Bates JA, Comerford A, Cetto R, Schroter CR (2016) Ineffective power mechanisms in pathological tracheas. J Biomech 49:2187–2192. https://doi.org/10.1016/j.jbiomech.2015.11.033
Si H, Jing H, Wang X, Qiu G (2017) Theoretical model for the performance of liquid ring pump based on the actual operating cycle. Int J Rotating. https://doi.org/10.1155/2017/3617321
Katzbauer B (1998) Properties and applications of xanthan gum. Polym Degrad Stabil 59:81–84. https://doi.org/10.1016/s0141-3910(97)00180-8
Tian M, Fang B, Jin L, Lu Y, Qiu X (2015) Rheological and drag reduction properties of hydroxypropyl xanthan gum solutions. Chinese J Chem Eng 23:1440–1446. https://doi.org/10.1016/j.cjche.2015.04.003
Liu F, Tang CH (2011) Cold, gel-like whey protein emulsions by microfluidisation emulsification: rheological properties and microstructures. Food Chem 127:1641–1647. https://doi.org/10.1016/j.foodchem.2011.02.031
Mandala IG, Savvas TP, Kostaropoulos AE (2004) Xanthan and locust bean gum influence on the rheology and structure of a white model-sauce. J Food Eng 64:335–342. https://doi.org/10.1016/j.jfoodeng.2003.10.018
Vieira JM, Andrade CCP, Santos TP, Okuro PK, Cunha RL (2020) Flaxseed gum-biopolymers interactions driving rheological behaviour of oropharyngeal dysphagia-oriented products. Food Hydrocolloid 111:106257. https://doi.org/10.1016/j.foodhyd.2020.106257
Escudier MP, Gouldson IW, Pereira AS, Pinho FT, Poole RJ (2001) On the reproducibility of the rheology of shear-thinning liquids. J Non-Newtonian Fluid Mech 97(2–3):99–124. https://doi.org/10.1016/S0377-0257(00)00178-6
Cao X, Zhao Z, Cheng L, Yin W (2016) Evaluation of a transparent analog fluid of digested sludge: xanthan gum aqueous solution. Procedia Environ Sci 31:735–742. https://doi.org/10.1016/j.proenv.2016.02.059
Abubakar A, Al-Wahaibi T, Al-Wahaibi Y, Al-Hashmi AR, Al-Ajmi A (2014) Roles of drag reducing polymers in single- and multi-phase flows. Chem Eng Res Des 92:2153–2181. https://doi.org/10.1016/j.cherd.2014.02.031
Roy AN, Benyahia L, Grisel M, Renou F (2016) Shear interfacial viscoelasticity of native and hydrophobically modified xanthan at oil/water interface. Food Hydrocolloid 61:887–894. https://doi.org/10.1016/j.foodhyd.2016.07.016
Barbieri SF, de Oliveira Petkowicz CR, de Godoy RCB, de Azeredo HCM, Franco CRC, Silveira JLM (2018) Pulp and jam of gabiroba (Campomanesia Xanthocarpa Berg) characterization and rheological properties. Food Chem 263:292–299. https://doi.org/10.1016/j.foodchem.2018.05.004
Nigmatullin R, Johns MA, Eichhorn SJ (2020) Hydrophobized cellulose nanocrystals enhance xanthan and locust bean gum network properties in gels and emulsions. Carbohyd Polym 250:116953. https://doi.org/10.1016/j.carbpol.2020.116953
Viturawong Y, Achayuthakan P, Suphantharika M (2008) Gelatinization and rheological properties of rice starch/xanthan mixtures: effects of molecular weight of xanthan and different salts. Food Chem 111:106–114. https://doi.org/10.1016/j.foodchem.2008.03.041
Sohn JI, Kim CA, Choi HJ, Jhon MS (2001) Drag-reduction effectiveness of xanthan gum in a rotating disk apparatus. Carbohyd Polym 45:61–68. https://doi.org/10.1016/S0144-8617(00)00232-0
Lee KH, Zhang K, Choi HJ (2010) Time dependence of turbulent drag reduction efficiency of polyisobutylene in kerosene. J Ind Eng Chem 16:499–502. https://doi.org/10.1016/j.jiec.2010.03.027
Jourdan L, Knapp Y, Oliver F, Guibergia JP (1998) The effect of drag-reducing polymer additives on wall-pressure fluctuations in turbulent channel flows. Eur J Mech - B/Fluids 17(1):105–136. https://doi.org/10.1016/S0997-7546(98)80054-X
Brostow W (2008) Drag reduction in flow: Review of applications, mechanism and prediction. J Ind Eng Chem 14:409–416. https://doi.org/10.1016/j.jiec.2008.07.001
Hong CH, Choi HJ, Kim JH (2008) Rotating disk apparatus for polymer-induced turbulent drag reduction. J Mech Sci Technol 22:1908–1913. https://doi.org/10.1007/s12206-008-0731-z
Lim ST, Hong CH, Choi HJ, Lai PY, Chan CK (2007) Polymer turbulent drag reduction near the theta point. Europhys Lett 80:58003. https://doi.org/10.1209/0295-5075/80/58003
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 51974304), the Independent Research Project of State Key Laboratory of Coal Resources and Safe Mining (Grant No. SKLCRSM21X002), the Natural Science Foundation of Hebei Province (Grant No. E2020402075), the State Key Laboratory Cultivation Base for Gas Geology and Gas Control (Henan Polytechnic University) (Grant No. WS2020A05), the Key Research and Development Program of Zibo City (Grant No. 2021XCCG0051) and the Qing Lan Project of Jiangsu Province.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, D., Kang, J., Liu, Y. et al. The effect of ultrahigh shear rate on the physical characteristics of xanthan gum. Polym. Bull. 80, 7641–7661 (2023). https://doi.org/10.1007/s00289-022-04423-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04423-8