Abstract
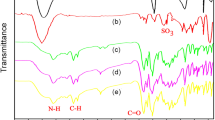
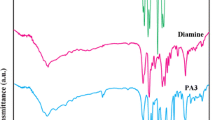
A new photosensitive and electroactive polyamide containing azobenzene in the side chain and triphenylamine unit in the main chain was prepared by reaction of 5-((4-(dimethylamino)phenyl)diazenyl)isophthalic acid (1) and 4-N-(4-aminophenyl)-4-N-phenyl benzene-1,4-diamine (2). A novel ternary (Mg–Zn–Al–) layered double hydroxide modified with calcon indicator (LDH-calcon) was prepared by a co-precipitation method. The solution intercalation technique was used to prepare nanocomposites from the polyamide and LDH-calcon in a solution of N,N-dimethylformamide. The LDH-calcon in polyamide 3 and 6 wt% was prepared. The photoisomerization of samples was monitored by 1H-NMR and UV–Vis spectroscopy. The absorption maxima (λmax) peak of 316 nm due to π–π∗ transition of trans-azobenzene chromophores modified to 254 nm UV irradiation. Cyclic voltammograms of the polyamide films on glassy carbon illustrated reversible redox around 0.6–0.86 V were enhanced by the addition of LDH-calcon. The thermal analysis exhibited enhanced T5 and T10 of polyamide with 3% LDH-calcon by about 76 °C and 174 °C, respectively, for nanocomposite containing 3 mass% of LDH-calcon and by about 26 °C and 15 °C for 6 mass%, respectively. Moreover, the char residues of nanocomposite increase as compared to neat polyamide.










Similar content being viewed by others
References
Hsiao SH, Chang YM, Chen HW, Liou GS (2006) Novel aromatic polyamides and polyimides functionalized with 4-tert-butyltriphenylamine groups. J Polym Sci Part A Polym Chem 44(15):4579–4592
Liou G-S, Chang C-W (2008) Highly stable anodic electrochromic aromatic polyamides containing N,N,N′,N′-tetraphenyl-p-phenylenediamine moieties: synthesis, electrochemical, and electrochromic properties. Macromolecules 41(5):1667–1674
Shabanian M, Ardeshir H, Haji-Ali S, Moghanian H, Hajibeygi M, Faghihi K, Khonakdar HA, Salimi H (2016) Efficient poly(methyl-ether-imide)/LDH nanocomposite derived from a methyl rich bisphenol: from synthesis to properties. Appl Clay Sci 123:285–291
Shabanian M, Basaki N, Khonakdar HA, Jafari SH, Hedayati K, Wagenknecht U (2014) Novel nanocomposites consisting of a semi-crystalline polyamide and Mg–Al LDH: morphology, thermal properties and flame retardancy. Appl Clay Sci 90:101–108
Sava I, Burescu A, Stoica I, Musteata V, Cristea M, Mihaila I, Pohoata V, Topala I (2015) Properties of some azo-copolyimide thin films used in the formation of photoinduced surface relief gratings. RSC Adv 5(14):10125–10133
Schab-Balcerzak E, Flakus H, Jarczyk-Jedryka A, Konieczkowska J, Siwy M, Bijak K, Sobolewska A, Stumpe J (2015) Photochromic supramolecular azopolyimides based on hydrogen bonds. Opt Mater 47:501–511
Clavier G, Ilhan F, Rotello VM (2000) Photochemical control of the macroconformation of polystyrene using azobenzene side chains. Macromolecules 33(25):9173–9175
He L-B, Mao H-P, Chao D-M, Zhang W-J (2007) Electroactive azo polyamide based oligoaniline: synthesis and characterization. Polym J 39(11):1172
Hu G, Zhao C, Zhang S, Yang M, Wang Z (2006) Low percolation thresholds of electrical conductivity and rheology in poly(ethylene terephthalate) through the networks of multi-walled carbon nanotubes. Polymer 47(1):480–488
Li H, Wang D, Fan H, Wang P, Jiang T, Xie T (2011) Synthesis of highly efficient C-doped TiO2 photocatalyst and its photo-generated charge-transfer properties. J Colloid Interface Sci 354(1):175–180
Shabanian M, Basaki N (2013) New photosensitive semi aramid/organoclay nanocomposite containing cinnamoyl groups: synthesis and characterization. Compos B Eng 52:224–232
Shabanian M, Basaki N (2017) Synthesis and characterization of new poly(ether-amide-imide)/amino-functionalized Fe3O4 nanocomposites. Polym Plast Technol Eng 56(9):966–973
Wang X, Zhang P, Chen Y, Luo L, Pang Y, Liu X (2011) Characterization of alignment correlation between LC molecules and chemical groups on/in the surface of polyimide films with biphenyl side chains. Macromolecules 44(24):9731–9737
Zulfiqar S, Ahmad Z, Ishaq M, Sarwar MI (2009) Aromatic–aliphatic polyamide/montmorillonite clay nanocomposite materials: synthesis, nanostructure and properties. Mater Sci Eng A 525(1–2):30–36
Mallakpour S, Dinari M (2013) Facile synthesis of nanocomposite materials by intercalating an optically active poly(amide-imide) enclosing (l)-isoleucine moieties and azobenzene side groups into a chiral layered double hydroxide. Polymer 54(12):2907–2916
Ding L, Russell TP (2007) A photoactive polymer with azobenzene chromophore in the side chains. Macromolecules 40(6):2267–2270
Leblond H, Barille R, Ahmadi-Kandjani S, Nunzi J, Ortyl E, Kucharski S (2009) Spontaneous formation of optically induced surface relief gratings. J Phys B At Mol Opt Phys 42(20):205401
Sava I, Hurduc N, Sacarescu L, Apostol I, Damian V (2013) Study of the nanostructuration capacity of some azopolymers with rigid or flexible chains. High Perform Polym 25(1):13–24
Iyi N, Ebina Y, Sasaki T (2011) Synthesis and characterization of water-swellable LDH (layered double hydroxide) hybrids containing sulfonate-type intercalant. J Mater Chem 21(22):8085–8095
Nhlapo N, Motumi T, Landman E, Verryn SM, Focke WW (2008) Surfactant-assisted fatty acid intercalation of layered double hydroxides. J Mater Sci 43(3):1033–1043
Coluccini C, Sporer I, Leuteritz A, Kuehnert I, Wang D-Y (2014) Layered double hydroxide: a new promising nanomaterial in energy application. Nanomater Energy 3(5):177–191
Goh K-H, Lim T-T, Dong Z (2008) Application of layered double hydroxides for removal of oxyanions: a review. Water Res 42(6–7):1343–1368
Alcantara A, Aranda P, Darder M, Ruiz-Hitzky E (2010) Bionanocomposites based on alginate–zein/layered double hydroxide materials as drug delivery systems. J Mater Chem 20(42):9495–9504
Li J, Fan Q, Wu Y, Wang X, Chen C, Tang Z, Wang X (2016) Magnetic polydopamine decorated with Mg–Al LDH nanoflakes as a novel bio-based adsorbent for simultaneous removal of potentially toxic metals and anionic dyes. J Mater Chem A 4(5):1737–1746
Yan L-g, Yang K, Shan R, Yan T, Wei J, Yu S, Yu H, Du B (2015) Kinetic, isotherm and thermodynamic investigations of phosphate adsorption onto core–shell Fe3O4@LDHs composites with easy magnetic separation assistance. J Colloid Interface Sci 448:508–516
Shabanian M, Kang N-J, Wang D-Y, Wagenknecht U, Heinrich G (2013) Synthesis of aromatic–aliphatic polyamide acting as adjuvant in polylactic acid (PLA)/ammonium polyphosphate (APP) system. Polym Degrad Stab 98(5):1036–1042
Tsai T-Y, Naveen B, Shiu W-C, Lu S-W (2014) An advanced preparation and characterization of the PET/MgAl-LDH nanocomposites. RSC Adv 4(49):25683–25691
Celebi S, Balan A, Epik B, Baran D, Toppare L (2009) Donor acceptor type neutral state green polymer bearing pyrrole as the donor unit. Org Electron 10(4):631–636
Wang Q, Tang SVY, Lester E, O'Hare D (2013) Synthesis of ultrafine layered double hydroxide (LDHs) nanoplates using a continuous-flow hydrothermal reactor. Nanoscale 5(1):114–117
Woo MA, Kim TW, Paek M-J, Ha H-W, Choy J-H, Hwang S-J (2011) Phosphate-intercalated Ca–Fe-layered double hydroxides: crystal structure, bonding character, and release kinetics of phosphate. J Solid State Chem 184(1):171–176
Ghasemian M, Kakanejadifard A, Karami T (2016) Synthesis, structural characterization, antimicrobial activities and theoretical investigations of some 4-(4-aminophenylsulfonyl)phenylimino)methyl)-4-(aryldiazenyl)phenol. Spectrochim Acta Part A Mol Biomol Spectrosc 168:190–198
Ghasemian M, Kakanejadifard A, Azarbani F, Zabardasti A, Kakanejadifard S (2014) Spectroscopy and solvatochromism studies along with antioxidant and antibacterial activities investigation of azo–azomethine compounds 2-(2-hydroxyphenylimino) methyl)-4-phenyldiazenyl) phenol. Spectrochim Acta Part A Mol Biomol Spectrosc 124:153–158
Yen HJ, Guo SM, Liou GS (2010) Synthesis and unexpected electrochemical behavior of the triphenylamine-based aramids with ortho-and para-trimethyl-protective substituents. J Polym Sci Part A Polym Chem 48(23):5271–5281
Wang X, Spörer Y, Leuteritz A, Kuehnert I, Wagenknecht U, Heinrich G, Wang D-Y (2015) Comparative study of the synergistic effect of binary and ternary LDH with intumescent flame retardant on the properties of polypropylene composites. RSC Adv 5(96):78979–78985
Kagunya W, Baddour-Hadjean R, Kooli F, Jones W (1998) Vibrational modes in layered double hydroxides and their calcined derivatives. Chem Phys 236(1–3):225–234
Chiang M-F, Chen E-C, Wu T-M (2012) Preparation, mechanical properties and thermal stability of poly (l-lactide)/γ-polyglutamate-modified layered double hydroxide nanocomposites. Polym Degrad Stab 97(6):995–1001
Kang N-J, Wang D-Y, Kutlu B, Zhao P-C, Leuteritz A, Wagenknecht U, Heinrich G (2013) A new approach to reducing the flammability of layered double hydroxide (LDH)-based polymer composites: preparation and characterization of dye structure-intercalated LDH and its effect on the flammability of polypropylene-grafted maleic anhydride/d-LDH composites. ACS Appl Mater Interfaces 5(18):8991–8997
Kozanecka-Szmigiel A, Konieczkowska J, Switkowski K, Antonowicz J, Trzebicka B, Szmigiel D, Schab-Balcerzak E (2016) Influence of supramolecular interactions on photoresponsive behavior of azobenzene poly (amide imide)s. J Photochem Photobiol A 318:114–123
Han Y-U, Lee W-S, Lee C-G, Park S-J, Kim K-W, Kim S-B (2011) Entrapment of Mg–Al layered double hydroxide in calcium alginate beads for phosphate removal from aqueous solution. Desalin Water Treat 36(1–3):178–186
Hajibeygi M, Shabanian M, Omidi-Ghallemohamadi M, Khonakdar HA (2017) Optical, thermal and combustion properties of self-colored polyamide nanocomposites reinforced with azo dye surface modified ZnO nanoparticles. Appl Surf Sci 416:628–638
Kakanejadifard A, Azarbani F, Zabardasti A, Rezayat A, Ghasemian M, Kakanejadifard S (2013) Spectroscopic and solvatochromism studies along with antioxidant and antibacterial activities investigation of 2-((2-mercaptophenylimino) methyl)-4-(phenyldiazenyl) phenol. Spectrochim Acta Part A Mol Biomol Spectrosc 114:404–409
Huang H-Y, Lee Y-T, Yeh L-C, Jian J-W, Huang T-C, Liang H-T, Yeh J-M, Chou Y-C (2013) Photoactively electroactive polyamide with azo group in the main chain via oxidative coupling polymerization. Polym Chem 4(2):343–350
Konieczkowska J, Janeczek H, Kozanecka-Szmigiel A, Schab-Balcerzak E (2016) Poly(amic acid)s and their poly(amide imide) counterparts containing azobenzene moieties: characterization, imidization kinetics and photochromic properties. Mater Chem Phys 180:203–212
Sahoo M, Sinha B, Marbaniang M, Naik D (2011) Degradation and mineralization of calcon using UV365/H2O2 technique: influence of pH. Desalination 280(1–3):266–272
Hsiao S-H, Liou G-S, Kung Y-C, Pan H-Y, Kuo C-H (2009) Electroactive aromatic polyamides and polyimides with adamantylphenoxy-substituted triphenylamine units. Eur Polym J 45(8):2234–2248
Hsiao S-H, Liou G-S, Kung Y-C, Lee Y-J (2010) Synthesis and characterization of electrochromic poly(amide–imide)s based on the diimide-diacid from 4,4′-diamino-4″-methoxytriphenylamine and trimellitic anhydride. Eur Polym J 46(6):1355–1366
Zhan T, Song Y, Tan Z, Hou W (2017) Electrochemical bisphenol A sensor based on exfoliated Ni2Al-layered double hydroxide nanosheets modified electrode. Sens Actuators B Chem 238:962–971
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Basaki, N., Kakanejadifard, A., Shabanian, M. et al. Synthesis and characterization of a new photosensitive and electroactive polyamide/LDH nanocomposite containing azo groups. Polym. Bull. 77, 6433–6448 (2020). https://doi.org/10.1007/s00289-019-03048-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-019-03048-8