Abstract
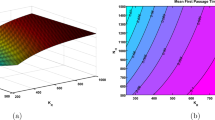
Seasonal variation affects the dynamics of many infectious diseases including influenza, cholera and malaria. The time when infectious individuals are first introduced into a population is crucial in predicting whether a major disease outbreak occurs. In this investigation, we apply a time-nonhomogeneous stochastic process for a cholera epidemic with seasonal periodicity and a multitype branching process approximation to obtain an analytical estimate for the probability of an outbreak. In particular, an analytic estimate of the probability of disease extinction is shown to satisfy a system of ordinary differential equations which follows from the backward Kolmogorov differential equation. An explicit expression for the mean (resp. variance) of the first extinction time given an extinction occurs is derived based on the analytic estimate for the extinction probability. Our results indicate that the probability of a disease outbreak, and mean and standard derivation of the first time to disease extinction are periodic in time and depend on the time when the infectious individuals or free-living pathogens are introduced. Numerical simulations are then carried out to validate the analytical predictions using two examples of the general cholera model. At the end, the developed theoretical results are extended to more general models of infectious diseases.








Similar content being viewed by others
References
Alam A, LaRocque RC, Harris JB, Vanderspurt C, Ryan ET, Qadri F, Calderwood SB (2005) Hyperinfectivity of human-passaged vibrio cholerae can be modeled by growth in the infant mouse. Infect Immun 73(10):6674–6679
Allen LJS, Lahodny GE Jr (2012) Extinction thresholds in deterministic and stochastic epidemic models. J Biol Dyn 6(2):590–611
Altizer S, Dobson A, Hosseini P, Hudson P, Pascual M, Rohani P (2006) Seasonality and the dynamics of infectious diseases. Ecol Lett 9(4):467–484
Andrews JR, Basu S (2011) Transmission dynamics and control of cholera in Haiti: an epidemic model. Lancet 377(9773):1248–1255
Athreya KB, Ney PE (1972) Branching processes. Springer, New York
Bacaër N (2007) Approximation of the basic reproduction number R0 for vector-borne diseases with a periodic vector population. Bull Math Biol 69(3):1067–1091
Bacaër N, Ait Dads EH (2014) On the probability of extinction in a periodic environment. J Math Biol 68(3):533–548
Bacaër N, Guernaoui S (2006) The epidemic threshold of vector-borne diseases with seasonality: the case of cutaneous leishmaniasis in Chichaoua Morocco. J Math Biol 53(3):421–436
Bani-Yaghoub M, Gautam R, Shuai Z, Van Den Driessche P, Ivanek R (2012) Reproduction numbers for infections with free-living pathogens growing in the environment. J Biol Dyn 6(2):923–940
Bertuzzo E, Casagrandi R, Gatto M, Rodriguez-Iturbe I, Rinaldo A (2010) On spatially explicit models of cholera epidemics. J R Soc Interface 7(43):321–333
Chao DL, Halloran ME, Longini IM (2011) Vaccination strategies for epidemic cholera in Haiti with implications for the developing world. Proc Natl Acad Sci 108(17):7081–7085
Chowdhury, A, Tanveer, S, Wang X (2020) Nonlinear two-point boundary value problems: applications to a cholera epidemic model. Proc Roy Soc A 476(2234):20190673
Codeço CT, Coelho FC (2006) Trends in cholera epidemiology. PLoS Med 3(1):e42
Codeço CT (2001) Endemic and epidemic dynamics of cholera: the role of the aquatic reservoir. BMC Infect Dis 1(1):1
Dangbé E, Irépran D, Perasso A, Békollé D (2018) Mathematical modelling and numerical simulations of the influence of hygiene and seasons on the spread of cholera. Math Biosci 296:60–70
Dorman KS, Sinsheimer JS, Lange K (2004) In the garden of branching processes. SIAM Rev 46(2):202–229
Dowell SF (2001) Seasonal variation in host susceptibility and cycles of certain infectious diseases. Emerg Infect Dis 7(3):369
Eisenberg MC, Kujbida G, Tuite AR, Fisman DN, J. H. Tien JH (2013a) Examining rainfall and cholera dynamics in Haiti using statistical and dynamic modeling approaches. Epidemics 5:197–207
Eisenberg MC, Shuai Z, Tien JH, Van den Driessche P (2013b) A cholera model in a patchy environment with water and human movement. Math Biosci 246(1):105–112
Emch M, Feldacker C, Islam MS, Ali M (2008) Seasonality of cholera from 1974 to 2005: a review of global patterns. Int J Health Geogr 7(1):31
Faruque SM, Albert MJ, Mekalanos JJ (1998) Epidemiology, genetics, and ecology of toxigenicvibrio cholerae. Microbiol Mol Biol Rev 62(4):1301–1314
Fox S, Miller J, Meyers L (2017) Seasonality in risk of pandemic influenza emergence. PLoS Comput Biol 13(10):e1005749
Grassly N, Fraser C (2006) Seasonal infectious disease epidemiology. Proc R Soc Lond B Biol Sci 273(1600):2541–2550
Harris TE (1963) The theory of branching processes. Springer, Berlin
Hartley DM, Morris JG Jr, Smith DL (2005) Hyperinfectivity: a critical element in the ability of V. cholerae to cause epidemics? PLoS Med 3(1):e7
He D, Wang X, Gao D, Wang J (2018) Modeling the 2016–2017 Yemen cholera outbreak with the impact of limited medical resources. J Theor Biol 451:80–85
Kalyani D, Shankar K (2016) Assessment and seasonal variations of communicable diseases: 3 year study. Int J Res Health Sci 4(4):1186–92
Kapp C (2009) Zimbabwe’s humanitarian crisis worsens. Lancet 373(9662):447
Koch R (1886) Further researches on cholera. Br Med J 1(1306):62
Koch R (1893) Über den augenblicklichen stand der bakteriologischen choleradiagnose. Z Hyg Infekt 14(1):319–338
Lahodny G Jr, Gautam R, Ivanek R (2015) Estimating the probability of an extinction or major outbreak for an environmentally transmitted infectious disease. J Biol Dyn 9(sup1):128–155
Liao S, Wang J (2011) Stability analysis and application of a mathematical cholera model. Math Biosci Eng 8(3):733–752
Luo J, Wang J, Wang H (2017) Seasonal forcing and exponential threshold incidence in cholera dynamics. Discrete Contin Dyn Syst B 22(6):2261–2290
Ma J, Ma Z (2006) Epidemic threshold conditions for seasonally forced SEIR models. Math Biosci Eng 3(1):161–172
Martinez ME (2018) The calendar of epidemics: seasonal cycles of infectious diseases. PLoS Pathog 14(11):e1007327
Mitchell C, Kribs C (2017) A comparison of methods for calculating the basic reproductive number for periodic epidemic systems. Bull Math Biol 79(8):1846–1869
Mode CJ (1971) Multitype branching processes theory and applications. Elsevier, New York
Mugero C, Hoque A (2001) Review of cholera epidemic in South Africa with focus on KwaZulu-Natal Province, Technical Report, KwaZulu-Natal Department of Health, Pietermaritzburg, South Africa
Mukandavire Z, Liao S, Wang J, Gaff H, Smith DL, Morris JG (2011) Estimating the reproductive numbers for the 2008–2009 cholera outbreaks in Zimbabwe. Proc Natl Acad Sci 108(21):8767–8772
Nelson EJ, Harris JB, Morris JG, Calderwood SB, Camilli A (2009) Cholera transmission: the host, pathogen and bacteriophage dynamic. Nat Rev Microbiol 7(10):693–702
Nipa KF, Allen LJS (2021) Chapter 1: The effect of demographic variability and periodic fluctuations on disease outbreaks in a vector-host epidemic model. In: Teboh-Ewungkem MI, Ngwa GA (eds) Infectious diseases and our planet, The mathematics of planet earth, vol 7. Springer Nature Switzerland, pp 15–35
Pascual M, Dobson A (2005) Seasonal patterns of infectious diseases. PLoS Med 2(1):e5
Posny D, Wang J (2014) Computing the basic reproductive numbers for epidemiological models in nonhomogeneous environments. Appl Math Comput 242:473–490
Posny D, Wang J (2014) Modelling cholera in periodic environments. J Biol Dyn 8(1):1–19
Rinaldo A, Bertuzzo E, Mari L, Righetto L, Blokesch M, Gatto M, Casagrandi R, Murray M, Vesenbeckh SM, Rodriguez-Iturbe I (2012) Reassessment of the 2010–2011 Haiti cholera outbreak and rainfall-driven multiseason projections. Proc Natl Acad Sci 109(17):6602–6607
Ruan S (2017) Modeling the transmission dynamics and control of rabies in China. Math Biosci 286:65–93
Sack DA, Sack SRB, Claire-Lise Chaignat MD (2006) Getting serious about cholera. N Engl J Med 355(7):649
Schmid-Hempel P, Frank SA (2007) Pathogenesis, virulence, and infective dose. PLoS Pathog 3(10):e147
Shuai Z, van den Driessche P (2013) Global stability of infectious disease models using Lyapunov functions. SIAM J Appl Math 73(4):1513–1532
Snow J (1855) On the mode of communication of Cholera. Edited by John Churchill. Second edn. London
Tian JP, Wang J (2011) Global stability for cholera epidemic models. Math Biosci 232(1):31–41
Tien JH, Earn DJ (2010) Multiple transmission pathways and disease dynamics in a waterborne pathogen model. Bull Math Biol 72(6):1506–1533
Tuite AR, Tien J, Eisenberg M, Earn DJ, Ma J, Fisman DN (2011) Cholera epidemic in Haiti, 2010: using a transmission model to explain spatial spread of disease and identify optimal control interventions. Ann Intern Med 154(9):593–601
Tuncer N, Martcheva M (2013) Modeling seasonality in avian influenza H5N1. J Biol Syst 21(04):1340004
Wang R-H, Jin Z, Liu Q-X, van de Koppel J, Alonso D (2012) A simple stochastic model with environmental transmission explains multi-year periodicity in outbreaks of avian flu. PLoS One 7(2):e28873
Wang W, Zhao X-Q (2008) Threshold dynamics for compartmental epidemic models in periodic environments. J Dyn Differ Eq 20(3):699–717
Wang X, Wang J (2015) Analysis of cholera epidemics with bacterial growth and spatial movement. J Biol Dyn 9(sup1):233–261
Wang X, Zhao X-Q (2017) A malaria transmission model with temperature-dependent incubation period. Bull Math Biol 79(5):1155–1182
Wang X, Zhao X-Q, Wang J (2018) A cholera epidemic model in a spatiotemporally heterogeneous environment. J Math Anal Appl 468(2):893–912
Wang X, Wang F-B (2019) Impact of bacterial hyperinfectivity on cholera epidemics in a spatially heterogeneous environment. J Math Anal Appl 480(2):123407
Wang F-B, Wang X (2021) A general multipatch cholera model in periodic environments. Discrete Continuous Dyn Syst B
Wesley CL, Allen LJS (2009) The basic reproduction number in epidemic models with periodic demographics. J Biol Dyn 3(2–3):116–129
Zhao X-Q (2017) Dynamical systems in population biology. Springer, New York
Yamazaki K, Wang X (2016) Global well-posedness and asymptotic behavior of solutions to a reaction-convection-diffusion cholera epidemic model. Discrete Continuous Dyn Syst B 21(4):1297
Yang C, Wang X, Gao D, Wang J (2017) Impact of awareness programs on cholera dynamics: two modeling approaches. Bull Math Biol 79(9):2109–2131
Acknowledgements
We thank the reviewers for their helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix
A ODE cholera model
The following assumptions about model (1) were made by Posny and Wang (2014b):
-
(H1)
\(f(t,0,0)=h(t,0,0)=0\);
-
(H2)
\(f(t, I, B)\ge 0\) for \((t, I, B)\in \mathbb {R}^3_{+}\);
-
(H3)
\(\frac{\partial f}{\partial I}\ge 0\), \(\frac{\partial f}{\partial B}\ge 0\), \(\frac{\partial h}{\partial I}\ge 0\) and \(\frac{\partial h}{\partial B}< 0\) for \((t, I, B)\in \mathbb {R}^3_{+}\);
-
(H4)
f and h are both concave functions in terms of I and B, for all \(t\ge 0\); i.e.,
$$\begin{aligned} D^2f = \begin{pmatrix} \frac{\partial ^2 f}{\partial I^2} &{} \frac{\partial ^2 f}{\partial I\partial B}\\ \frac{\partial ^2 f}{\partial B\partial I} &{} \frac{\partial ^2 f}{\partial B^2} \end{pmatrix}, \quad D^2h = \begin{pmatrix} \frac{\partial ^2 h}{\partial I^2} &{} \frac{\partial ^2 h}{\partial I\partial B}\\ \frac{\partial ^2 h}{\partial B\partial I} &{} \frac{\partial ^2 h}{\partial B^2} \end{pmatrix} \end{aligned}$$are negative semidefinite for \((t, I, B)\in \mathbb {R}^3_{+}\).
-
(H5)
\(f(t, 0, B)>0\) if \(B>0\); \(h(t, I, 0)>0\) if \(I>0\).
-
(H6)
For simplicity, write \(f=f(t, x_1, x_2)\) and \(h=h(t, x_1,x_2)\). There exists \(\epsilon >0\), such that for \(0<x_1,x_2<\epsilon \), we have
$$\begin{aligned} u(t, x_1,x_2)\ge \left. \left[ u+\sum _{i=1}^2\frac{\partial u}{\partial x_i}+\frac{1}{2}\sum _{i, j=1}^2\frac{\partial ^2 u}{\partial x_i\partial x_j}\right] \right| _{(t,x_1,x_2)=(t,0,0),}\quad {\text {for }} u=f, h. \end{aligned}$$
The global stability of the DFE when \({\mathcal {R}}_0<1\) and uniform persistence of the solution of model (1) when \({\mathcal {R}}_0>1\) were verified in (Posny and Wang 2014b, Theorems 3, 5) and they are summarized as follows:
Theorem 1
-
(i)
If \({\mathcal {R}}_0<1\), the DFE of model (1) is globally asymptotically stable, and for any solution x(t) of model (1),
$$\begin{aligned} \lim _{t\rightarrow \infty }x(t)=(N,0,0,0)^T. \end{aligned}$$ -
(ii)
Suppose that assumptions (H1)-(H6) hold. If \(R_0>1\), then the solutions of system (1) are uniformly persistent and the system admits at least one positive \(\omega \)-periodic solution.
B general epidemic model
For the more general linearized ODE model in (23),
the conditions on F and V in Bacaër and Ait Dads (2014) for all \(t\ge 0\) are
-
(H1)
F(t) is nonnegative with at least one entry strictly positive;
-
(H2)
\(F(t) - V(t)\) is irreducible;
-
(H3)
F(t) and V(t) are piecewise continuous and \(\omega \)-periodic in t;
-
(H4)
\(-V(t)\) is cooperative (i.e., off-diagonal coefficients are nonnegative) and there exists \(c>0\) such that \(V_{ii}(t)\ge c\) for \(t\ge 0\).
C multiple infections
The branching process approximation of the probability of an outbreak in (13) shows good agreement with the time-nonhomogeneous process of the stochastic cholera Models 1 and 2 when initial conditions are either \((I(\tau ),B(\tau ))=(2,2)\) or (0, 6) Fig. 9.
Probabilities of an outbreak, \({\mathbb {P}}_{outbreak}(2,2,\tau )\) and \({\mathbb {P}}_{outbreak}(0,6,\tau )\), Eq. (13), are graphed for the stochastic cholera Models 1 (solid black curve) and 2 (dashed red curve). The proportion of \(2\times 10^4\) sample paths that result in an outbreak (\(I+0.25 B\ge 200)\) for the time-nonhomogeneous process at \(\tau =0,365/12, 365/6,\ldots ,365\) are marked by blue circles. Parameter values are the same as in Figs. 3, 6
Rights and permissions
About this article
Cite this article
Allen, L.J.S., Wang, X. Stochastic models of infectious diseases in a periodic environment with application to cholera epidemics. J. Math. Biol. 82, 48 (2021). https://doi.org/10.1007/s00285-021-01603-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00285-021-01603-4
Keywords
- Nonhomogeneous stochastic process
- Branching process
- Seasonality
- Cholera
- Epidemic
- Extinction probability
- Extinction time