Abstract
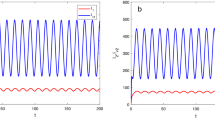
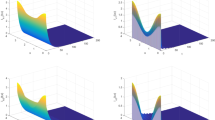
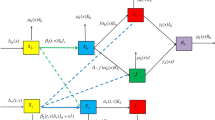
In this paper, we introduce a reaction–diffusion malaria model which incorporates vector-bias, spatial heterogeneity, sensitive and resistant strains. The main question that we study is the threshold dynamics of the model, in particular, whether the existence of spatial structure would allow two strains to coexist. In order to achieve this goal, we define the basic reproduction number \(R_{i}\) and introduce the invasion reproduction number \({\hat{R}}_{i}\) for strain \(i (i=1,2)\). A quantitative analysis shows that if \(R_{i}<1\), then disease-free steady state is globally asymptotically stable, while competitive exclusion, where strain i persists and strain j dies out, is a possible outcome when \(R_{i}>1>R_{j}\) \((i\ne j, i,j=1,2)\), and a unique solution with two strains coexist to the model is globally asymptotically stable if \(R_{i}>1\), \({\hat{R}}_{i}>1\). Numerical simulations reinforce these analytical results and demonstrate epidemiological interaction between two strains, discuss the influence of resistant strains and study the effects of vector-bias on the transmission of malaria.










Similar content being viewed by others
References
Abboubakar H, Buonomo B, Chitnis N (2016) Modelling the effects of malaria infection on mosquito biting behaviour and attractiveness of humans. Ric di Mat 65(1):329–346
Atkinson MP, Su Z, Alphey N, Alphey LS, Coleman PG, Wein LM (2007) Analyzing the control of mosquito-borne diseases by a dominant lethal genetic system. Proc Natl Acad Sci USA 104(22):9540–9545
Bai Z, Peng R, Zhao XQ (2018) A reaction–diffusion malaria model with seasonality and incubation period. J Math Biol 77(1):201–228
Buonomo B, Vargas-De-León C (2012) Stability, and bifurcation analysis of a vector-bias model of malaria transmission. Math Biosci 242(1):59–67
Cantrell RS, Cosner C (2003) Spatial ecology via reaction–diffusion equations. Wiley, Hoboken
CDC (2018) https://www.cdc.gov/malaria/malaria_worldwide/impact.html
Chamchod F, Britton NF (2011) Analysis of a vector-bias model on malaria transmission. Bull Math Biol 73(3):639–657
Chitnis N, Pemberton-Ross P, Yukich J, Hamainza B, Smith TA (2019) Theory of reactive interventions in the elimination and control of malaria. Malar J 18(1):266
Diekmann O, Heesterbeek JAP, Metz JAJ (1990) On the definition and the computation of the basic reproduction ratio R\(_0\) in models for infectious-diseases in heterogeneous populations. J Math Biol 28(4):365–382
Dreessche P, Watmough J (2002) Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math Biosci 180(1–2):29–48
Esteva L, Gumel AB, Vargas-De-León C (2009) Qualitative study of transmission dynamics of drug-resistant malaria. Math Comput Model 50(3–4):611–630
Feng Z, Qiu Z, Sang Z, Lorenzo C, Glasser J (2013) Modeling the synergy between HSV-2 and HIV and potential impact of HSV-2 therapy. Math Biosci 245(2):171–187
Fitzgibbon WE, Morgan JJ, Webb G (2017) An outbreak vector-host epidemic model with spatial structure: the 2015–2016 Zika outbreak in Rio De Janeiro. Theor Biol Med Model 14(1):7
Forouzannia F, Gumel A (2015) Dynamics of an age-structured two-strain model for malaria transmission. Appl Math Comput 250:860–886
Ge J, Kim KI, Lin Z, Zhu H (2015) A SIS reaction–diffusion–advection model in a low-risk and high-risk domain. J Differ Equ 259(2015):5486–5509
Guo Z, Wang FB, Zou X (2012) Threshold dynamics of an infective disease model with a fixed latent period and non-local infections. J Math Biol 65(6–7):1387–1410
Hagenaars TJ, Donnelly CA, Ferguson NM (2004) Spatial heterogeneity and the persistence of infectious diseases. J Theor Biol 229(3):349–359
Hale JK, Waltman P (1989) Persistence in infinite-dimensional systems. SIAM J Math Anal 20(2):388–395
Hethcote HW, Ark JWV (1987) Epidemiological models for heterogeneous populations: proportionate mixing, parameter estimation, and immunization programs. Math Biosci 84(1):85–118
Hetzel M, Chitnis N (2020) Reducing malaria transmission with reactive focal interventions. Lancet 395(10233):1317–1319
Kim S, Masud MA, Cho G, Jung IH (2017) Analysis of a vector-bias effect in the spread of malaria between two different incidence areas. J Theor Biol 419:66–76
Kingsolver JG (1987) Mosquito host choice and the epidemiology of malaria. Am Nat 130(6):811–827
Lacroix R, Mukabana RW, Clement Gouagna L, Jacob KC (2005) Malaria infection increases attractiveness of humans to mosquitoes. PLOS Biol 3(9):e298
Li J, Zou X (2009) Modeling spatial spread of infectious diseases with a fixed latent period in a spatially continuous domain. Bull Math Biol 71(8):2048–2079
Liang X, Zhang L, Zhao XQ (2017) Basic reproduction ratios for periodic abstract functional differential equations (with application to a spatial model for lyme disease). J Dyn Differ Equ 31:1247–1278
Lou Y, Zhao XQ (2011) A reaction–diffusion malaria model with incubation period in the vector population. J Math Biol 62(4):543–568
Macdonald G (1952) The analysis of equilibrium in malaria. Trop Dis Bull 49(9):813–829
Macdonald G (1957) The epidemiology and control of malaria. Oxford University Press, London
Magal P, Zhao XQ (2005) Global attractors and steady states for uniformly persistent dynamical systems. SIAM J Math Anal 37(1):251–275
Magal P, Webb G, Wu Y (2018) On a vector-host epidemic model with spatial structure. Nonlinearity 31(12):5589–5614
Magal P, Webb G, Wu Y (2019) On the basic reproduction number of reaction–diffusion epidemic models. SIAM J Appl Math 79(1):284–304
Martin JRH (1976) Nonlinear operators and differential equations in Banach spaces. Wiley-Interscience, New York
Martin RH, Smith HL (1990) Abstract functional differential equations and reaction–diffusion systems. Trans Am Math Soc 321(1):1–44
Ménard D, Khim N, Beghain J, Adegnika AA, Geertruyden JPV (2016) A worldwide map of plasmodium falciparum K13-propeller polymorphisms. N Engl J Med 347(25):2453–2464
Mischaikow K, Smith H, Thieme RH (1995) Asymptotically autonomous semiflows: chain recurrence and Lyapunov functions. Trans Am Math Soc 347(5):1669–1685
Nosten F, White NJ (2007) Artemisinin-based combination treatment of falciparum malaria. Am J Trop Med Hyg 77(6Suppl):181
Oisaemi I, Babatunde A, Adeniyi O, Oluseye B (2017) Quality of artemisinin-based antimalarial drugs marketed in Nigeria. Trans R Soc Trop Med Hyg 111(2):90–96
Reiker T, Chitnis N, Smith T (2019) Modelling reactive case detection strategies for interrupting transmission of plasmodium falciparum malaria. Malar J 18(1):259
Robert V, Macintyre K, Keating J, Trape JF, Duchemin JB, Warren M, Beier JC (2003) Malaria transmission in urban sub-Saharan Africa. Am J Trop Med Hyg 68(2):169–176
Ross R (1911) The prevention of malaria. John Murray, London
Ross R (1916) An application of the theory of probabilities to the study of a priori pathometry. Part I. Proc R Soc Lond A 92(638):204–230
Smith HL (1995) Monotone dynamical systems: an introduction to the theory of competitive and cooperative systems, vol 41. Mathematical surveys and monographs. American Mathematical Society, Providence
Smith HL, Zhao XQ (2001) Robust persistence for semidynamical systems. Nonlinear Anal Theory Methods Appl 47(9):6169–6179
Smoller J (1994) Shock waves and reaction diffusion equations. Springer, New York
Tabachnick WJ (2010) Challenges in predicting climate and environmental effects on vector-borne disease episystems in a changing world. J Exp Biol 213(6):946
Tamsin EL, Penny MA (2019) Identifying key factors of the transmission dynamics of drug-resistant malaria. J Theor Biol 462:210–220
Thieme HR (1992) Convergence results and Poincare–Bendixson trichotomy for asymptotically autonomous differential equations. J Math Biol 30(7):755–763
Thieme HR (2009) Spectral bound and reproduction number for infinite-dimensional population structure and time heterogeneity. SIAM J Appl Math 70:188–211
Tumwiine J, Hove-Musekwa DS, Nyabadza F (2014) A mathematical model for the transmission and spread of drug sensitive and resistant malaria strains within a human population. ISRN Biomath 4:1–12
Tuncer N, Martcheva M (2012) Analytical and numerical approaches to coexistence of strains in a two-strain SIS model with diffusion. J Biol Dyn 6(2):406–439
Vargas-De-León C (2012) Global analysis of a delayed vector-bias model for malaria transmission with incubation period in mosquitoes. Math Biosci Eng 9(1):165–174
Wang J, Chen Y (2020) Threshold dynamics of a vector-borne disease model with spatial structure and vector-bias. Appl Math Lett 100:106052
Wang W, Zhao XQ (2012) Basic reproduction numbers for reaction–diffusion epidemic models. SIAM J Appl Dyn Syst 11(4):1652–1673
Wang W, Zhao XQ (2015) Spatial invasion threshold of Lyme disease. SIAM J Appl Math 75(3):1142–1170
Wang X, Zhao XQ (2017) A periodic vector-bias malaria model with incubation period. SIAM J Appl Math 77(1):181–201
WHO (2018) https://www.who.int/malaria/media/world-malaria-report-2018/zh/
Wu J (1996) Theory and applications of partial functional differential equations. Springer, New York
Wu R, Zhao XQ (2019) A reaction–diffusion model of vector-borne disease with periodic delays. J Nolinear Sci 29:29–64
Xu Z, Zhang Y (2015) Traveling wave phenomena of a diffusive and vector-bias malaria model. Commun Pure Appl Anal 14(3):923–940
Xu Z, Zhao XQ (2013) A vector-bias malaria model with incubation period and diffusion. Discrete Continuous Dyn Syst Ser B 17(7):2615–2634
Yeung S, Pongtavornpinyo W, Hastings IM, Mills AJ, White NJ (2004) Antimalarial drug resistance, artemisinin-based combination therapy, and the contribution of modeling to elucidating policy choices. Am J Trop Med Hyg 71(2):179–186
Zhang X, Zhang Y (2018) Spatial dynamics of a reaction–diffusion cholera model with spatial heterogeneity. Discrete Continuous Dyn Syst Ser B 23(6):2625–2640
Zhao L, Wang ZC, Ruan S (2020) Dynamics of a time-periodic two-strain SIS epidemic model with diffusion and latent period. Nonlinear Anal-Real 51:102966
Zhao XQ (2012) Global dynamics of a reaction and diffusion model for lyme disease. J Math Biol 65(4):787–808
Zhao XQ (2017) Dynamical systems in population biology. Springer, London
Acknowledgements
We thank the editors and reviewers for their comments and suggestions, which help improve the presentation of the paper. The authors are grateful for the support and useful comments provided by Professor Xiaoqiang Zhao (Memorial University of Newfoundland). This research is supposed by National Science Foundation of China (grant number: 11971013, 11571170).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
1.1 The details of assumption \(N(t,x)\equiv N(x)\)
Here, based on the research of Bai et al. (2018), we will take \(b_{h}\) as a typical birth rate function, i.e., \(b_{h}=b_{h}(N_{h})=b_{0}e^{-N_{h}/K(x)}\), where \(b_{0}>0\) is the maximal individual birth rate of human, and K(x), standing for the local carrying capacity, is supposed to be a positive function of location x.
We prove this result in several steps.
Step 1. It is easy to see that for any \(\phi \in C({\bar{\varOmega }},{\mathbb {R}}_{+})\), system (1)–(2) has a unique positive solution \(N(t,x,{\phi })\) on \([0,\infty )\times {\bar{\varOmega }}\) (see, Martin and Smith 1990, Corollary 5), with \(N(0,x,\phi )=\phi \). Define \(P_{t}:C({\bar{\varOmega }},{\mathbb {R}}_{+})\rightarrow C({\bar{\varOmega }},{\mathbb {R}}_{+})\) by
It then follows that \(\{P_{t}\}_{t\ge 0}\) is a semifolw on \(C({\bar{\varOmega }},{\mathbb {R}}_{+})\).
For any \(\phi \in C({\bar{\varOmega }},{\mathbb {R}}_{+})\) with \(\phi \gg 0\) one easily finds that \(N(t,x;\phi )>0\) for \(t\ge 0\) and \(x\in \varOmega \). Fix \(k\in (0,1)\) and let \(w(t,x)=N(t,x;k\phi )-kN(t,x;\phi )\). Then \(w(0,x)=0\) for \(x\in \varOmega \). For \(t>0\), w(t, x) satisfies
Let \(f(t,N(t,x;\phi ))=b_{h}(N(t,x;\phi ))N(t,x;\phi )\), then (39) can be written as
where
Let U(t, s), \(t\ge s\ge 0\), be the evolution operator of the linear parabolic equation
Following Smith (1995, Theorem 7.4.1), one easily sees that U(t, s), \(t> s\ge 0\), is strongly positive, i.e., for any \(\varphi >0\), \(U(t,s)\varphi \gg 0\). By the formula of variation of constants, we have
Since \(N(t,\cdot ;\phi )>0\), \(t\ge 0\), when \(\phi \gg 0\) and f(t, N) is strictly subhomogeneous in N, we have \(h(s,\cdot )>0\) and hence \(w(t,\cdot )>0\) for \(t>0\). Therefore, \(P_{t}(k\phi )>kP_{t}(\phi )\) for each \(t>0\), so \(P_{t}\) is strictly subhomogeneous.
Step 2. For each \(t\ge 0\), the Frechet derivative \(DP_{t}(t)=\varPhi (t)\), where \(\varPhi (t)\) is the semigroup generated by the linear equation \(\frac{\partial N_{h}(t,x)}{\partial t}=d\varDelta N_{h}(t,x)+g(x,0)N_{h}(t,x)\) with \(\frac{\partial N_{h}(t,x)}{\partial \nu }=0\), where \(g(x,N_{h}(t,x))=b_{h}(N_{h}(t,x))-d_{h}\). It then follows that \(r(D(P_{t}(0)))=e^{\lambda _{0}(d,g(x,0))t}\), where \(\lambda _{0}(d,g(x,0))\) is the principle eigenvalue of \(d\varDelta \phi +g(x,0)\phi =\lambda \phi \) with \(\frac{\partial \phi }{\partial \nu }=0\).
Step 3. In the case, where \(r(D(P_{t}(0)))>1\), i.e., \(\lambda _{0}(d,g(0,x))>0\), apply the Zhao (2017, Theorem 2.3.4) to the time-one map \(P_{1}\). There exists a unique positive fixed point of \(P_{1}\), which is assumed to be N(x).
Step 4. Since system (1)–(2) is autonomous, then \(P_{1}(P_{t}(N(x)))=P_{t+1}(N(x))=P_{t}(P_{1}(N(x)))=P_{t}(N(x))\). The uniqueness of positive fixed point of \(P_{1}\) implies that \(P_{t}(N(x))=N(x)\), \(\forall t\ge 0\), that is, N(x) is a steady state of (1).
Therefore, it is reasonable to assume that \(N(t,x)\equiv N(x)\).
1.2 The derivation of P(A|B)
Rights and permissions
About this article
Cite this article
Shi, Y., Zhao, H. Analysis of a two-strain malaria transmission model with spatial heterogeneity and vector-bias. J. Math. Biol. 82, 24 (2021). https://doi.org/10.1007/s00285-021-01577-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00285-021-01577-3