Abstract
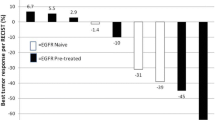
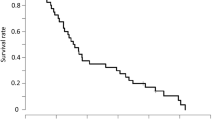
Purpose: In a phase II trial, the activity of carzelesin, a cyclopropylpyrroloindole prodrug analog, was assessed. Patients and methods: Carzelesin was used as second- or third-line chemotherapy in patients with breast, ovarian, head and neck cancer and non-Hodgkin's lymphoma, and as first-line chemotherapy in patients with colorectal and gastric cancer and melanoma. The drug was given as a bolus infusion at a 4-weekly dose of 150 μg/m2. A total of 140 patients were entered and a total of 285 courses were administered. Results: In general, the compound was well tolerated. Myelotoxicity was the most common toxicity. Grade 3 and 4 leukopenia was observed in 18.6% of the courses, neutropenia in 20.3%, thrombocytopenia in 16.2% and anemia in 8.7%. Double nadirs were seen in a total of 41 courses for neutrophils, in 40 for leukocytes and in 3 for platelets. Non-hematological toxicity was very mild. Only one partial response in a patient with melanoma was seen. Conclusions: At this dose and schedule carzelesin did not yield activity in the types of tumors studied.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 8 December 1999 / Accepted: 10 April 2000
Rights and permissions
About this article
Cite this article
Pavlidis, N., Aamdal, S., Awada, A. et al. Carzelesin phase II study in advanced breast, ovarian, colorectal, gastric, head and neck cancer, non-Hodgkin's lymphoma and malignant melanoma: a study of the EORTC early clinical studies group (ECSG). Cancer Chemother Pharmacol 46, 167–171 (2000). https://doi.org/10.1007/s002800000134
Issue Date:
DOI: https://doi.org/10.1007/s002800000134