Abstract
Purpose
For patients with locally advanced non-small-cell lung cancer (LA-NSCLC) that progressed after definitive chemoradiotherapy (CRT) and durvalumab consolidation therapy, no subsequent standard treatment exists. The type of treatment selected for each timing of disease progression and its efficacy have not been investigated.
Methods
We retrospectively enrolled patients with LA-NSCLC or inoperable NSCLC that progressed after definitive CRT and durvalumab consolidation therapy at 15 Japanese institutions. Patients were classified into the following: Early Discontinuation group (disease progression within 6 months after durvalumab initiation), Late Discontinuation group (disease progression from 7 to 12 months after durvalumab initiation), and Accomplishment group (disease progression from 12 months after durvalumab initiation).
Results
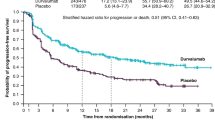
Altogether, 127 patients were analyzed, including 50 (39.4%), 42 (33.1%) and 35 (27.5%) patients from the Early Discontinuation, Late Discontinuation, and Accomplishment groups, respectively. Subsequent treatments were Platinum plus immune checkpoint inhibitors (ICI) in 18 (14.2%), ICI in 7 (5.5%), Platinum in 59 (46.4%), Non-Platinum in 35 (27.6%), and tyrosine kinase inhibitor in 8 (6.3%) patients. In the Early Discontinuation, Late Discontinuation, and Accomplishment groups, 4 (8.0%), 7 (16.7%), and 7 (20.0%) patients were receiving Platinum plus ICI; 21 (42.0%), 22 (52.4%), and 16 (45.7%) were receiving Platinum, and 20 (40.0%), 8 (19.0%), and 7 (20.0%) were receiving Non-Platinum, respectively. No significant difference in progression-free survival was observed in the timing of disease progression.
Conclusion
In patients with LA-NSCLC hat progressed after definitive CRT and durvalumab consolidation therapy, subsequent treatment may change depending on the timing of disease progression.

Similar content being viewed by others
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Marino P, Preatoni A, Cantoni A (1995) Randomized trials of radiotherapy alone versus combined chemotherapy and radiotherapy in stages IIIa and IIIb nonsmall cell lung cancer. A meta-analysis. Cancer 76(4):593–601. https://doi.org/10.1002/1097-0142(19950815)76:4%3c593::aid-cncr2820760409%3e3.0.co;2-n
Pritchard RS, Anthony SP (1996) Chemotherapy plus radiotherapy compared with radiotherapy alone in the treatment of locally advanced, unresectable, non-small-cell lung cancer. A meta-analysis. Ann Intern Med 125(9):723–729. https://doi.org/10.7326/0003-4819-125-9-199611010-00003
Aupérin A, Le Péchoux C, Rolland E, Curran WJ, Furuse K, Fournel P, Belderbos J, Clamon G, Ulutin HC, Paulus R, Yamanaka T, Bozonnat MC, Uitterhoeve A, Wang X, Stewart L, Arriagada R, Burdett S, Pignon JP (2010) Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 28(13):2181–2190. https://doi.org/10.1200/jco.2009.26.2543
Spigel DR, Faivre-Finn C, Gray JE, Vicente D, Planchard D, Paz-Ares L, Vansteenkiste JF, Garassino MC, Hui R, Quantin X, Rimner A, Wu YL, Özgüroğlu M, Lee KH, Kato T, de Wit M, Kurata T, Reck M, Cho BC, Senan S, Naidoo J, Mann H, Newton M, Thiyagarajah P, Antonia SJ (2022) Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol 40(12):1301–1311. https://doi.org/10.1200/jco.21.01308
Imai H, Kaira K, Mori K, Ono A, Akamatsu H, Taira T, Yoshino R, Kenmotsu H, Saitoh J, Harada H, Naito T, Murakami H, Tomizawa Y, Matsuura M, Saito R, Nakajima T, Yamada M, Takahashi T (2015) Comparison of platinum combination re-challenge therapy and docetaxel monotherapy in non-small cell lung cancer patients previously treated with platinum-based chemoradiotherapy. Springerplus 4:152. https://doi.org/10.1186/s40064-015-0929-3
Giaj Levra M, Cotte FE, Corre R, Calvet C, Gaudin AF, Penrod JR, Grumberg V, Jouaneton B, Jolivel R, Assie JB, Chouaid C (2020) Immunotherapy rechallenge after nivolumab treatment in advanced non-small cell lung cancer in the real-world setting: a national data base analysis. Lung Cancer 140:99–106. https://doi.org/10.1016/j.lungcan.2019.12.017
Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro CJ, Faivre-Finn C, Reck M, Vansteenkiste J, Spigel DR, Wadsworth C, Melillo G, Taboada M, Dennis PA, Özgüroğlu M (2018) Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 379(24):2342–2350. https://doi.org/10.1056/NEJMoa1809697
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Freites-Martinez A, Santana N, Arias-Santiago S, Viera A (2021) Using the common terminology criteria for adverse events (CTCAE—version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr (Engl Ed) 112(1):90–92. https://doi.org/10.1016/j.ad.2019.05.009
Giaccone G, Ferrati P, Donadio M, Testore F, Calciati A (1987) Reinduction chemotherapy in small cell lung cancer. Eur J Cancer Clin Oncol 23(11):1697–1699. https://doi.org/10.1016/0277-5379(87)90452-4
Wakuda K, Kenmotsu H, Naito T, Akamatsu H, Ono A, Shukuya T, Nakamura Y, Tsuya A, Murakami H, Takahashi T, Endo M, Nakajima T, Yamamoto N (2015) Efficacy of rechallenge chemotherapy in patients with sensitive relapsed small cell lung cancer. Am J Clin Oncol 38(1):28–32. https://doi.org/10.1097/COC.0b013e318286907b
Kim YH, Goto K, Yoh K, Niho S, Ohmatsu H, Kubota K, Saijo N, Nishiwaki Y (2008) Performance status and sensitivity to first-line chemotherapy are significant prognostic factors in patients with recurrent small cell lung cancer receiving second-line chemotherapy. Cancer 113(9):2518–2523. https://doi.org/10.1002/cncr.23871
Schoenfeld AJ, Antonia SJ, Awad MM, Felip E, Gainor J, Gettinger SN, Hodi FS, Johnson ML, Leighl NB, Lovly CM, Mok T, Perol M, Reck M, Solomon B, Soria JC, Tan DSW, Peters S, Hellmann MD (2021) Clinical definition of acquired resistance to immunotherapy in patients with metastatic non-small-cell lung cancer. Ann Oncol 32(12):1597–1607. https://doi.org/10.1016/j.annonc.2021.08.2151
Acknowledgements
We thank the patients and their families as well as all the investigators.
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
TH: Conceptualization, methodology, formal analysis, investigation, resources, data curation, writing—original draft, writing—review and editing, visualization, and project administration. RA: Conceptualization, methodology, investigation, resources, data curation, writing—review and editing, supervision, and project administration. HT, RS, YK, AH, TS, TT, JS, MS, YT, TS, AM, SU, and YT-K: Investigation, resources, data curation, and writing—review and editing. NY and MN: Writing—review and editing, supervision, and project administration.
Corresponding author
Ethics declarations
Conflict of interest
Tsukasa Hasegawa reports honoraria from AstraZeneca, Chugai Pharmaceutical, and Eli Lilly and Company. Ryo Ariyasu reports honoraria from AstraZeneca, Chugai Pharmaceutical, and Bristol-Myers Squibb. Hisashi Tanaka reports honoraria from AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Chugai Pharmaceutical Ono Pharmaceutical, and Pfizer. Yosuke Kawashima reports honoraria from AstraZeneca, Chugai Pharmaceutical, Eli Lilly and Company, and Taiho Pharmaceutical. Atsushi Horiike reports receiving grants from BeiGene, Chugai Pharmaceutical, Daiichi Sankyo, Merck Sharp & Dohme, and Nippon Kayaku and honoraria from AstraZeneca, Bristol-Myers Squibb, Chugai Pharmaceutical, Eli Lilly and Company, EP-SOGO, Kyowa Hakko Kirin, Merck Sharp & Dohme, Novartis, and Taiho Pharmaceutical. Takehiro Tozuka reports honoraria from AstraZeneca and Chugai Pharmaceutical. Yuichi Tambo reports honoraria from AstraZeneca, Chugai Pharmaceutical, Merck Sharp & Dohme, Takeda Pharmaceutical, and Taiho Pharmaceutical. Tomoaki Sonoda reports honoraria from AstraZeneca, Chugai Pharmaceutical, Kyowa Hakko Kirin, Ono Pharmaceutical, and Taiho Pharmaceutical. Shinya Uematsu reports honoraria from AstraZeneca, Chugai Pharmaceutical, Eli Lilly and Company, Kyowa Hakko Kirin, Nippon Kayaku, Novartis, Ono Pharmaceutical, Pfizer, and Takeda Pharmaceutical. Yuko Tsuchiya-Kawano reports honoraria from Bristol-Myers Squibb, Chugai Pharmaceutical, Kyowa Hakko Kirin, Ono Pharmaceutical, and Takeda Pharmaceutical. Noriko Yanagitani reports honoraria from AstraZeneca, Bayer Yakuhin, Bristol-Myers Squibb, Chugai Pharmaceutical, Eli Lilly and Company, Ono Pharmaceutical, Pfizer, and Takeda Pharmaceutical and receiving payment for expert testimony from Chugai Pharmaceutical. Makoto Nishino reports honoraria from AbbVie, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Chugai Pharmaceutical, Daiichi Sankyo, Eli Lilly and Company, Janssen, Merck Sharp & Dohme, Nippon Kayaku, Novartis, Merck Serono, Ono Pharmaceutical, Pfizer, Takeda Pharmaceutical, and Taiho Pharmaceutical. All other authors have stated that they have no conflicts of interest.
Ethical approval
The studies included in the analysis were conducted in accordance with the Declaration of Helsinki and the good clinical practice guidelines and were approved by the institutional review board of The Jikei University Daisan Hospital.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hasegawa, T., Ariyasu, R., Tanaka, H. et al. Subsequent treatment for locally advanced non-small-cell lung cancer that progressed after definitive chemoradiotherapy and consolidation therapy with durvalumab: a multicenter retrospective analysis (TOPGAN 2021-02). Cancer Chemother Pharmacol 92, 29–37 (2023). https://doi.org/10.1007/s00280-023-04547-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-023-04547-2