Abstract
Background
To evaluate the efficacy of docetaxel, cisplatin, and 5-fluorouracil as combination chemoradiotherapy (DCF-RT) for cervical esophageal cancer (CEC), we performed a retrospective analysis of CEC patients treated by DCF-RT at a single institution.
Methods
We conducted a single-center retrospective study. Twenty-one patients with CEC who underwent DCF-RT between 1999 and 2017 at our institute were included in this study. Chemotherapy consisted of intravenous docetaxel at 50 mg/m2 on day 1, intravenous CDDP at 60 mg/m2 on day 1, and intravenous 5-FU at 600 mg/m2 on days 1–4, repeated every 4 weeks for two cycles. Among the 21 patients, six were irradiated using three-dimensional conformal RT (3D- conformal RT) and 15 were treated using intensity-modulated RT (IMRT) consisting of 60 Gy in 30 fractions.
Results
The median follow-up period was 49.6 months (range 4.6–97.6). The overall complete response (CR) and local CR rates were 61.9% and 81.0% for all patients, and 76.9% and 84.6% for patients without hypopharyngeal and/or thoracic esophageal invasion, respectively. The 3-year overall survival (OS), progression-free survival (PFS), and local failure-free survival (LFFS) rates were 79.6, 52.4, and 74.7%, respectively. Grade 3–4 leucopenia developed in 12 patients (70.6%), neutropenia developed in 13 patients (81.2%), and mucositis developed in 2 patients (9.5%). There were no treatment-related deaths.
Conclusions
The 3-year OS and LFFS of patients who underwent DCF-RT were higher than those in the previous studies. Although the high rate of myelosuppression requires careful management, DCF-RT is a safe and effective modality for CEC.
Similar content being viewed by others
Introduction
Cervical esophageal cancer (CEC) is uncommon and accounts for less than 5% of all cases of esophageal cancer [1]. As CEC is often diagnosed locally, with advanced disease infiltrating nearby anatomical structures, such as the hypopharynx, trachea, thyroid, recurrent laryngeal nerves, pharyngo–laryngo–esophagectomy (PLE) is often required for curative resection. Therefore, definitive chemoradiation (CRT) is the standard treatment modality recommended by the current guidelines [2, 3]. Although the 3-year overall survival (OS) rate was reported to be less than 40% [4, 5], a 3-year OS of greater than 50% was recently reported [6, 7]. In these previous studies, the most commonly employed regimens with concurrent radiation therapy were a combination of cisplatin (CDDP) and 5-FU. CDDP and 5-FU therapy (CF therapy) is the standard regimen for esophageal cancer [8,9,10]. We previously reported that docetaxel, cisplatin, and 5-fluorouracil as combination chemoradiotherapy (DCF-RT) led to a high complete response (CR) rate and favorable prognosis compared with standard chemoradiotherapy for advanced esophageal cancer [11]. However, the clinical results of DCF-RT for CEC were unclear. To evaluate the efficacy of DCF-RT for CEC, we performed a retrospective analysis of CEC patients who received DCF-RT at a single institution.
Patients and methods
Patients
This retrospective study was approved by the ethics committee of the Graduate School of Medicine, Gunma University. Written informed consent was obtained from all patients before the treatment. Between 1999 and 2017, 57 patients underwent chemoradiotherapy for cervical esophageal cancer (CEC) at our institute. Of these 57 patients, 26 received docetaxel, cisplatin, and 5-fluorouracil as combination chemoradiotherapy (DCF- RT). In total, 21 patients were enrolled, and five patients were excluded because of simultaneous radiation for a skip lesion in the thoracic esophagus (n = 2), synchronous laryngeal cancer (n = 1), adenocarcinoma (n = 1), or insufficient response evaluation (n = 1). The flowchart presented in Fig. 1 outlines this study. In this study, CEC was defined as a tumor located between the esophageal orifice and the sternal notch based on the Japanese Classification of Esophageal Cancer, 11th edition [12]. Patients with hypopharyngeal and/or thoracic esophageal invasion were included. Hospital patient records were reviewed for tumor characteristics and patient outcomes. All cases were confirmed histologically as squamous cell carcinoma. Tumor stage and disease grade were classified according to the 7th edition of the TNM classification of the International Union Against Cancer (UICC) [13]. The tumor stage was determined conventionally using computed tomography (CT) and positron emission tomography (PET) –CT of the neck, chest, and abdomen, endoscopic ultrasonography (EUS), endoscopy, and esophagography.
Treatment
Patients received concurrent radiotherapy and chemotherapy for 6 weeks after the diagnostic procedures. Chemotherapy consisted of intravenous docetaxel at 50 mg/m2 on day 1, intravenous CDDP at 60 mg/m2 on day 1, and intravenous 5-FU at 600 mg/m2 on days 1–4 (DCF), repeated every 4 weeks for two cycles. Among the 21 patients, six were irradiated using 3-dimensional conformal radiotherapy (3D-conformal RT) at a daily dose of 1.8–2.0 Gy, and 15 patients were treated using intensity-modulated radiation therapy (IMRT) at a daily dose of 2.0 Gy to the gross tumor volume. For 3D-conformal RT, the initial 40–46 Gy was delivered using anterior–posterior opposed fields. The field of radiation included the 5 cm margin with the primary tumor located craniocaudally and 2 cm beyond the radial margins. Prophylactic irradiation to the regional lymph nodes was performed. The boost dose of 10–20 Gy employing the shrinking field technique was delivered using oblique parallel opposed fields to avoid the spinal cord. For IMRT, the gross tumor volume (GTV) was measured using pretreatment CT, PET-CT, and endoscopy data. The clinical target volume (CTV) primary was defined as the GTV primary with 2–2.5 cm superior and inferior margins. The CTV node was defined as the GTV node. Supraclavicular (level IV) and superior mediastinal lymph node regions were contoured as CTV prophylactic in all cases; treatment of the upper neck (levels Ib-III and V) was at the discretion of the radiation oncologist. The planning target volume (PTV) was expanded from the CTV by 0.5–1.0 cm to account for setup variations and uncertainties. The setup margins were selected according to intrafractional motion. We used two types of IMRT procedures: the first employed a simultaneous integrated boost technique over the entire period, whereas the other applied an IMRT boost after completion of 3D-conformal RT. For both groups, 60 Gy in 30 fractions was delivered to PTVprimary and PTVnode. For PTVprophylactic, 48 Gy in 30 fractions was delivered in the simultaneous integrated boost group, whereas 20 Gy in 10 fractions was delivered to PTVprimary and PTVnode using IMRT after the initial 40 Gy delivered by 3D-conformal RT in the boost IMRT group. The simultaneous integrated boost (n = 4) and IMRT boost (n = 11) groups were both entered into the IMRT group in this analysis. In this study, 6 patients who received an IMRT boost were treated by one cycle of intravenous DCF and 5-FU at 700 mg/m2 on days 29–32, and intravenous CDDP at 70 mg/m2 on day 29 (FP), because the IMRT boost for these patients was administered at an affiliated hospital. Five of the six patients were treated by boost IMRT with thermal therapy (once a week for 2 weeks).
Response evaluation
Standard clinical measurements and radiological examinations were used to assess the tumor response according to RECIST. The treatment response of the primary lesion (non-target lesion) was evaluated according to the Japanese Classification of Esophageal Cancer, 11th edition [12]. One month after the completion of treatment, the first evaluation of the initial tumor response was carried out. The second evaluation was carried out after more than 4 weeks from the first evaluation. Endoscopy was repeated to confirm primary complete response (CR).
Toxicity
Toxicities were assessed using the Common Terminology Criteria for Adverse Events v4.0.
Follow-up
Patients were assessed every 3 months after treatment completion for the first 2 years, and every 6 months after that. CR was confirmed by endoscopy, biopsy specimens, CT, and PET-CT.
Statistical analysis
Locoregional failure was defined as the persistence or recurrence of the primary tumor or regional lymph nodes. Distant metastatic failure was defined as metastasis to any site beyond the primary tumor and regional lymph nodes. Overall survival (OS) was calculated from the start of treatment to the last follow-up or death. Progression-free survival (PFS) was calculated from the start day of treatment to the date of disease progression or any cause of death. Local failure-free survival (LFFS) was calculated from the start day of treatment to the date of local failure or any cause of death. Kaplan–Meier curves were generated for OS, PFS, and LFFS. All analyses were performed using R version 2.13.0 (The R Foundation for Statistical Computing, Vienna, Austria) statistical software.
Results
Patient characteristics
The patient characteristics are detailed in Table 1. The median follow-up period was 49.6 months (range 4.6–97.6). The relative dose intensity in the first cycle of DCF was 99.5%. Excluding the six patients who were administered FP for the second cycle of chemotherapy, four patients received full-dose chemotherapy, eight patients received DCF at 80% for the second cycle, and two patients received no chemotherapy for the second cycle. Thus, the relative dose intensity in the second cycle of DCF was 74.0%.
Treatment outcome
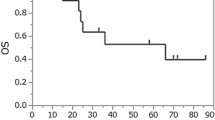
After initial response analysis, the overall CR rate was 61.9% for all patients and 76.9% for patients without hypopharyngeal and/or thoracic esophageal invasion (pure CEC). The local CR rate was 81.0% for all patients and 84.6% for patients with pure CEC. The 3-year OS, PFS, and LFFS rates were 79.6% (95% confidence interval [CI] = 53.9%–91.9%), 52.4% (95% CI = 29.7%–70.9%), and 74.7% (95% CI = 49.2%–88.7%), respectively (Fig. 2a, b, c).
Failure pattern and salvage treatment
Ten patients had recurrence of any type at the time of analysis. In these patients, the initial failure pattern was locoregional failure in 4 (40%) and distant metastases in 6 (60%). Among the four patients with locoregional failure, three patients (75%) underwent salvage treatment without severe complications. One patient underwent endoscopic submucosal dissection, two patients underwent PLE, and the remaining one patient had no further anticancer treatment. Among the six patients with distant metastasis, four patients had upper cervical node recurrence outside the prophylactic irradiation area and one patient had paraaortic abdominal node recurrence. The remaining two patients had distant organ metastases [brain (n = 1), lung (n = 1)]. During the study period, nine patients were alive without disease, three patients died due to progressive disease, and three patients of noncancerous causes.
Toxicities
The toxicity profile is presented in Table 2. The most commonly observed grade 3 and 4 acute toxicities were leucopenia, neutropenia, and mucositis. There were no treatment-related deaths.
Regarding late toxicity, we observed grade 1 radiation pneumonitis in one patient, grade 1 plural effusion in one patient, and grade 2 plural effusion in one patient. Among the local failure-free patients, we noted grade 1 esophageal stenosis in one patient and grade 2 esophageal stenosis in one patient.
Discussion
In this study, the 3-year OS, PFS, and LFFS rates were 79.6, 52.4, and 74.7%, respectively, for patients with CEC who underwent DCF-RT. The overall CR rate was 61.9% and the local CR rate was 81.0% for all patients. To the best of our knowledge, this is the first report of the clinical results of DCF-RT for cervical esophageal cancer.
The results from the previous studies on the outcome of CEC treated by CRT are summarized in Table 3 [4,5,6,7, 14,15,16]. The 3-year OS was approximately 40% before 2015; however, in recent studies, several groups reported an improved 3-year OS range from 53.6 to 66.6%. In our study, the overall CR rate was 61.9%, which was lower than that in recent studies [6, 7], but our study included more patients with T4 disease (66%) and hypopharyngeal or thoracic esophageal extension. For patients with T4 CEC, the local CR rate was 78.5% (11 of 14), which was superior to that in a previous study [6].
Although advanced disease was included, the 3-year OS and LFFS in our study were higher than those in the previous studies. One reason may be the same beneficial effects of DCF-RT for CEC as for head and neck cancer [17] and lower esophageal cancer [18]. Conformal radiotherapy (3D-conformal RT and IMRT) may also have positively affected the OS in our study. Conformal radiotherapy techniques have been reported to increase local tumor control [19]. Ito et al. compared the efficacy of IMRT and 3D-conformal RT for CEC, and found that the 3-year OS of IMRT was better than that of 3D-CRT, although the local control rate of IMRT and 3D-CRT was not significantly different [7]. They speculated that IMRT can reduce the high-dose irradiated area in normal tissue, thereby improving the salvage rate and prognosis. These findings support our salvage rate of 75% for patients with locoregional failure without severe complications.
The dominant initial failure pattern was distant metastases (60%) in our study. Our local control rate of over 80% may have changed the failure pattern to distant metastasis. However, the main initial site of failure for CEC after CRT is controversial [6], [14]. One possible reason for this contradiction (locoregional vs. distant) is the difference in patient background and therapeutic strategies.
The incidence rates of grade 3 and 4 leukopenia (70.6%), neutropenia (80.2%), mucositis (9.5%), and dermatitis (4.7%) were similar to those in studies [17], [20]. As the relative dose intensity decreased to 74.0% in the second DCF cycle from 99.5% in the first DCF cycle, mainly due to severe myelosuppression or late recovery from myelosuppression, prevention and precise management of these hematological toxicities may improve the efficacy of DCF-RT for CEC. However, whether prophylactic use of long-acting G-CSF agents increases the dose intensity remains unclear.
This study has several limitations. First, there was a selection bias because this was a retrospective single-institution analysis with a relatively small sample size. Second, the heterogeneity of radiation procedures limited the power of our study to analyze the pure effects of DCF using the unified radiation strategy. Due to its rarity, further multicenter, large-population-based investigations are needed to confirm the effects of advances in chemoradiotherapy for CEC.
In conclusion, DCF-RT for CEC demonstrated a satisfactory CR rate, and the 3-year OS and LFFS of patients who underwent DCF-RT were higher than those in the previous studies. Although the high rate of myelosuppression requires careful management, DCF-RT is a safe and effective modality for CEC.
References
Sombeck MD, Parsons JT et al (1994) Management of cervical esophageal carcinoma. Semin Radiat Oncol 4:179–191. https://doi.org/10.1053/SRAO00400179
Kuwano H, Nishimura Y, Oyama T et al (2015) Guidelines for diagnosis and treatment of carcinoma of the Esophagus April 2012 edited by the Japan esophageal society. Esophagus. https://doi.org/10.1007/s10388-014-0465-1
Ajani JA, D’Amico TA, Almhanna K et al (2015) Esophageal and esophagogastric junction cancers, version 1.2015. J Natl Compr Canc Netw 13:194–227
Yamada K, Murakami M, Okamoto Y et al (2006) Treatment results of radiotherapy for carcinoma of the cervical esophagus. Acta Oncol (Madr) 45:1120–1125. https://doi.org/10.1080/02841860600609768
Zhang P, Xi M, Zhao L et al (2015) Clinical efficacy and failure pattern in patients with cervical esophageal cancer treated with definitive chemoradiotherapy. Radiother Oncol 116:257–261. https://doi.org/10.1016/j.radonc.2015.07.011
Zenda S, Kojima T, Kato K et al (2016) Multicenter phase 2 study of cisplatin and 5-fluorouracil with concurrent radiation therapy as an organ preservation approach in patients with squamous cell carcinoma of the cervical esophagus. Int J Radiat Oncol Biol Phys 96:976–984. https://doi.org/10.1016/j.ijrobp.2016.08.045
Ito M, Kodaira T, Tachibana H et al (2017) Clinical results of definitive chemoradiotherapy for cervical esophageal cancer: comparison of failure pattern and toxicities between intensity-modulated radiotherapy and 3-dimensional conformal radiotherapy. Head Neck 39:2406–2415. https://doi.org/10.1002/hed.24909
Minsky BD, Pajak TF, Ginsberg RJ et al (2002) INT 0123 (radiation therapy oncology Group 94-05) phase III trial of combined-Modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol 20:1167–1174. https://doi.org/10.1200/JCO.2002.20.5.1167
Kato K, Eguchi Nakajima T, Ito Y et al (2013) Phase II study of concurrent chemoradiotherapy at the dose of 50.4 Gy with elective nodal irradiation for stage II-III esophageal carcinoma. Jpn J Clin Oncol 43:608–615. https://doi.org/10.1093/jjco/hyt048
Ishida K, Ando N, Yamamoto S et al (2004) Phase II study of cisplatin and 5-fluorouracil with concurrent radiotherapy in advanced squamous cell carcinoma of the esophagus: a Japan esophageal oncology group (JEOG)/Japan clinical oncology group trial (JCOG9516). Jpn J Clin Oncol 34:615–619. https://doi.org/10.1093/jjco/hyh107
Miyazaki T, Sohda M, Tanaka N et al (2015) Phase I/II study of docetaxel, cisplatin, and 5-fluorouracil combination chemoradiotherapy in patients with advanced esophageal cancer. Cancer Chemother Pharmacol 75:449–455
Japan Esophageal Society JE (2017) Japanese classification of esophageal cancer, 11th edition: part II and III. Esophagus 14:37–65. https://doi.org/10.1007/s10388-016-0556-2
Sobin L, Gospodarowicz M WC (2009) TNM Classification of Malignant Tumours, 7th Edition. In: Wiley
Cao C, Luo J, Gao L et al (2015) Definitive radiotherapy for cervical esophageal cancer. Head Neck 37:151–155. https://doi.org/10.1002/hed.23572
Huang SH, Lockwood G, Brierley J et al (2008) Effect of concurrent high-dose cisplatin chemotherapy and conformal radiotherapy on cervical esophageal cancer survival. Int J Radiat Oncol 71:735–740. https://doi.org/10.1016/j.ijrobp.2007.10.022
Zhao L, Zhou Y, Mu Y et al (2017) Patterns of failure and clinical outcomes of definitive radiotherapy for cervical esophageal cancer. Oncotarget. https://doi.org/10.18632/oncotarget.15665
Katori H, Tsukuda M (2005) Comparison of induction chemotherapy with docetaxel, cisplatin, and 5-fluorouracil (TPF) followed by radiation vs concurrent chemoradiotherapy with TPF in patients with locally advanced squamous cell carcinoma of the head and neck. Clin Oncol 17:148–152. https://doi.org/10.1016/j.clon.2004.09.013
Tamaki Y, Hieda Y, Nakajima M et al (2018) Concurrent chemoradiotherapy with docetaxel, cisplatin, and 5-fluorouracil improves survival of patients with advanced esophageal cancer compared with conventional concurrent chemoradiotherapy with cisplatin and 5-fluorouracil. J Cancer 9:2765–2772. https://doi.org/10.7150/jca.23456
Bedford JL, Viviers L, Guzel Z et al (2000) A quantitative treatment planning study evaluating the potential of dose escalation in conformal radiotherapy of the oesophagus. Radiother Oncol 57:183–193
Higuchi K, Komori S, Tanabe S et al (2014) Definitive chemoradiation therapy with docetaxel, cisplatin, and 5-fluorouracil (DCF-R) in advanced esophageal cancer: a phase 2 trial (KDOG 0501-P2). Int J Radiat Oncol Biol Phys 89:872–879. https://doi.org/10.1016/j.ijrobp.2014.03.030
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest associated with this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sakai, M., Sohda, M., Saito, H. et al. Docetaxel, cisplatin, and 5-fluorouracil combination chemoradiotherapy for patients with cervical esophageal cancer: a single-center retrospective study. Cancer Chemother Pharmacol 83, 1121–1126 (2019). https://doi.org/10.1007/s00280-019-03835-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-019-03835-0