Abstract
Purpose
To assess the efficacy and safety of regorafenib versus rechallenge chemotherapy in previously treated mCRC patients in third-line setting.
Materials and methods
The data of 104 patients diagnosed with mCRC enrolled from 2010 to 2017 in six oncology centers were analyzed. Tumor treatment options were obtained from follow-up and treatment files. Rechallenge chemotherapy was identified as the re-use of the regimen which was previously administered to patients in one of the therapy lines and obtained disease control, these were the patients whose disease did not progress within 3 months.
Results
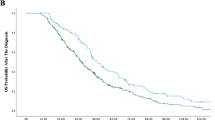
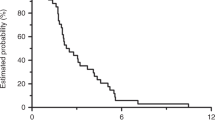
A total of 104 patients had received previously two lines of chemotherapy regimens for mCRC. Of these, 73 patients with mCRC who received regorafenib and 31 those who received rechallenge chemotherapy in third-line therapy were analyzed. Overall survival was better with rechallenge than it was with regorafenib (HR 0.29 95% CI 0.16–0.54, p < 0.001). Median OS was 12.0 months (95% CI 8.1–15.9) in rechallenge versus 6.6 months (95% CI 6.0–7.3) in regorafenib group (p < 0.001). Progression-free survival in the rechallenge group showed a higher median value of 9.16 months (95% CI 7.15–11.18) versus with that recorded in the regorafenib group of 3.41 months (95% CI 3.01–3.82), in favor of rechallenge chemotherapy. The most common adverse events of regorafenib was liver function test abnormality and hand–foot syndrome. Although grade 3 or 4 adverse events were similar, non-hematologic toxicities were more common than those of rechallenge.
Conclusions
Rechallenge is still a valuable option against regorafenib in patients who achieved disease control in one of the first two lines of therapy. Even though mCRC patients treated with regorafenib benefited clinically from this treatment, we revealed that chemotherapy rechallenge compared to regorafenib was more effective in the third-line treatment for mCRC patients.


Similar content being viewed by others
References
Shah MA, Renfro LA, Allegra CJ et al (2016) Impact of patient factors on recurrence risk and time dependency of oxaliplatin benefit in patients with colon cancer: analysis from modern-era adjuvant studies in the Adjuvant Colon Cancer End Points (ACCENT) database. J Clin Oncol 34:843–853
Heinemann V, von Weikersthal LF, Decker T et al (2014) FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 15:1065–1075
Venook AP, Niedzwiecki D, Lenz HJ et al (2017) Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial. JAMA 317:2392–2401
Giantonio BJ, Catalano PJ, Meropol NJ et al (2007) Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 5:1539–1544
Grothey A, Van Cutsem E, Sobrero A et al (2013) Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381(9863):303–312
Li J, Qin S, Xu R et al (2015) Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 16:619–629
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Arnold D, Prager GW, Quintela A et al (2018) Beyond second-line therapy in patients with metastatic colorectal cancer: a systematic review. Ann Oncol 29(4):835–856
Patel AK, Duh MS, Barghout V et al (2018) Real-world treatment patterns among patients with colorectal cancer treated with trifluridine/tipiracil and regorafenib. Clin Colorectal Cancer 17(3):531–539
Matsuda C, Honda M, Tanaka C et al (2016) Multicenter randomized phase II clinical trial of oxaliplatin reintroduction as a third- or later-line therapy for metastatic colorectal cancer-biweekly versus standard triweekly XELOX (The ORION Study). Int J Clin Oncol 21(3):566–572
Hartmann JT, Oechsle K, Jager E et al (2004) Prospective multicenter phase II study of irinotecan as third-line therapy in metastatic colorectal cancer and progression after bolus and infusional 5-fluorouracil. Anticancer Drugs 15(5):473–477
Suenaga M, Mizunuma N, Matsusaka S et al (2015) Phase II study of reintroduction of oxaliplatin for advanced colorectal cancer in patients previously treated with oxaliplatin and irinotecan: rE-OPEN study. Drug Des Devel Ther 9:3099–3108
Gebbia V, Del Prete S, Borsellino N et al (2006) Efficacy and safety of cetuximab/irinotecan in chemotherapy-refractory metastatic colorectal adenocarcinomas: a clinical practice setting, multicenter experience. Clin Colorectal Cancer 5(6):422–428
Lievre A, Samalin E, Mitry E et al (2009) Bevacizumab plus FOLFIRI or FOLFOX in chemotherapy-refractory patients with metastatic colorectal cancer: a retrospective study. BMC Cancer 9:347
Chaix M, Vincent J, Lorgis V, Ghiringhelli F (2014) FOLFIRINOX bevacizumab is a promising therapy for chemorefractory metastatic colorectal cancer. Oncology 87(3):148–158
Larsen FO, Boisen MK, Fromm AL, Jensen BV (2012) Capecitabine and bevacizumab in heavily pre-treated patients with advanced colorectal cancer. Acta Oncol 51(2):231–233
Jime´nez-Fonseca P, Solis MP, Garrido M et al (2015) Gemcitabine plus capecitabine (Gem-Cape) biweekly in chemorefractory metastatic colorectal cancer. Clin Transl Oncol 17(5):384–392
Yoshida M, Takagane A, Miyake Y et al (2016) A phase II study of third-line combination chemotherapy with bevacizumab plus S-1 for metastatic colorectal cancer with mutated KRAS (SAVIOR study). Oncology 91(1):24–30
Kwon HC, Oh SY, Lee S, Kim SH, Kim HJ (2007) Bevacizumab plus infusional 5-fluorouracil, leucovorin and irinotecan for advanced colorectal cancer that progressed after oxaliplatin and irinotecan chemotherapy: a pilot study. World J Gastroenterol 13(46):6231–6235
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Köstek, O., Hacıoğlu, M.B., Sakin, A. et al. Regorafenib or rechallenge chemotherapy: which is more effective in the third-line treatment of metastatic colorectal cancer?. Cancer Chemother Pharmacol 83, 115–122 (2019). https://doi.org/10.1007/s00280-018-3713-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-018-3713-6