Abstract
Purpose
To determine the recommended dose and antitumor activity of single-agent elisidepsin as a 24-h intravenous (i.v.) infusion fortnightly [biweekly, d1 and 15 every 4 weeks (q4wk); Arm A, dose-intensity strategy] or as a 3-h i.v. infusion weekly (d1, 8, 15 and 22 q4wk; Arm B, dose-density strategy) in adult patients with unresectable, locally advanced or metastatic pretreated esophageal, gastroesophageal junction and gastric cancer.
Methods
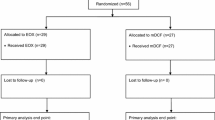
Patients were randomized to one of two elisidepsin dosing schedules. Phase Ib starting doses were 8.0 mg flat dose (FD) in Arm A and 3.0 mg FD in Arm B. Phase II subsequently explored antitumor activity of both dosing schedules at the respective recommended doses.
Results
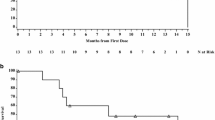
Forty-four patients received elisidepsin: 12 in stage Ib and 32 in stage II. The recommended doses were defined as 10 mg FD (Arm A) and 3.75 mg FD (Arm B). Both schedules were well tolerated. Most adverse events were mild or moderate, reversible and predictable with no meaningful differences between schedules. The pharmacokinetic profiles of both schedules were similar to those reported previously in patients with solid tumors treated with a comparable dose. An interim analysis found tumor control in one patient receiving elisidepsin fortnightly, and in none given elisidepsin weekly; patient accrual was therefore discontinued due to lack of efficacy.
Conclusions
Both schedules at the recommended doses presented an acceptable safety profile, but lack of response means that we do not recommend further evaluation of single-agent elisidepsin as chemotherapy for unresectable, locally advanced or metastatic gastroesophageal cancer.

Similar content being viewed by others
References
Hamann MT, Otto CS, Scheuer PJ, Dunbar DC (1996) Kahalalides: bioactive peptides from a marine mollusk Elysia rufescens and Its algal diet Bryopsis sp. (1). J Org Chem 61(19):6594–6600
Sparidans RW, Stokvis E, Jimeno JM, Lopez-Lazaro L, Schellens JH, Beijnen JH (2001) Chemical and enzymatic stability of a cyclic depsipeptide, the novel, marine-derived, anti-cancer agent kahalalide F. Anticancer Drugs 12(7):575–582
Faircloth G, Cuevas C (2006) Kahalalide F and ES285: potent anticancer agents from marine molluscs. Prog Mol Subcell Biol 43:363–379
Provencio M, Sanchez A, Gasent J, Gomez P, Rosell R (2009) Cancer treatments: can we find treasures at the bottom of the sea? Clin Lung Cancer 10(4):295–300
Herrero AB, Astudillo AM, Balboa MA, Cuevas C, Balsinde J, Moreno S (2008) Levels of SCS7/FA2H-mediated fatty acid 2-hydroxylation determine the sensitivity of cells to antitumor PM02734. Cancer Res 68(23):9779–9787
Molina-Guijarro JM, Macias A, Garcia C, Munoz E, Garcia-Fernandez LF, David M, Nunez L, Martinez-Leal JF, Moneo V, Cuevas C, Lillo MP, Villalobos Jorge C, Valenzuela C, Galmarini CM (2011) Irvalec inserts into the plasma membrane causing rapid loss of integrity and necrotic cell death in tumor cells. PLoS ONE 6(4):e19042
Kiraly A, Varadi T, Hajdu T, Ruhl R, Galmarini CM, Szollosi J, Nagy P (2013) Hypoxia reduces the efficiency of elisidepsin by inhibiting hydroxylation and altering the structure of lipid rafts. Mar Drugs 11(12):4858–4875. doi:10.3390/md11124858
Ling YH, Aracil M, Jimeno J, Perez-Soler R, Zou Y (2009) Molecular pharmacodynamics of PM02734 (elisidepsin) as single agent and in combination with erlotinib; synergistic activity in human non-small cell lung cancer cell lines and xenograft models. Eur J Cancer 45(10):1855–1864. doi:10.1016/j.ejca.2009.03.003
Salazar R, Cuadra C, Gil-Martin M, Vandermeeren A, Alfaro V, Coronado C (2012) Complete and sustained objective response per RECIST to Irvalec (PM02734) in undifferentiated large cell esophageal adenocarcinoma: a case report and a review of the literature. Case Rep Oncol 5(2):354–358. doi:10.1159/000341104000341104
Salazar R, Jones RJ, Oaknin A, Crawford D, Cuadra C, Hopkins C, Gil M, Coronado C, Soto-Matos A, Cullell-Young M, Iglesias Dios JL, Evans TR (2012) A phase I and pharmacokinetic study of elisidepsin (PM02734) in patients with advanced solid tumors. Cancer Chemother Pharmacol 70(5):673–681. doi:10.1007/s00280-012-1951-6
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. doi:10.1016/j.ejca.2008.10.026
Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10(1):1–10
Enzinger PC, Ilson DH, Kelsen DP (1999) Chemotherapy in esophageal cancer. Semin Oncol 26(5 Suppl 15):12–20
Ilson DH (2008) Esophageal cancer chemotherapy: recent advances. Gastrointest Cancer Res 2(2):85–92
Bilici A (2014) Treatment options in patients with metastatic gastric cancer: current status and future perspectives. World J Gastroenterol WJG 20(14):3905–3915. doi:10.3748/wjg.v20.i14.3905
Ratain MJ, Geary D, Undevia SD, Coronado C, Alfaro V, Iglesias JL, Schilsky RL, Miguel-Lillo B (2015) First-in-human, phase I study of elisidepsin (PM02734) administered as a 30-min or as a 3-h intravenous infusion every three weeks in patients with advanced solid tumors. Invest New Drugs 33(4):901–910. doi:10.1007/s10637-015-0247-1
Acknowledgments
This study was funded by Pharma Mar, S.A. The study was supported at the Glasgow Centre by the Experimental Cancer Medicine Centre (ECMC), funded by Cancer Research UK and the Chief Scientist Office, Scotland. R.D. Petty’s current affiliation is Ninewells Hospital and Medical School, University of Dundee, Dundee, United Kingdom. B. Miguel-Lillo’s current affiliation is SGS Exprimo NV, Mechelen, Belgium.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
R. D. Petty has received funding from Roche and AstraZeneca, remuneration from Eli Lilly and Merck, and has had a consultant/advisory role for Eli Lilly and AstraZeneca. J. P. Metges has received remuneration from Amgen, Sanofi and Merck. P. Bohan is an employee of PharmaMar. J. L. Iglesias Dios is a shareholder and employee of PharmaMar. B. Miguel-Lillo and G. Laus are shareholders and former employees of PharmaMar. All other authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Petty, R., Anthoney, A., Metges, JP. et al. Phase Ib/II study of elisidepsin in metastatic or advanced gastroesophageal cancer (IMAGE trial). Cancer Chemother Pharmacol 77, 819–827 (2016). https://doi.org/10.1007/s00280-016-2991-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-2991-0