Abstract
Purpose
This study aimed to evaluate the safety of neoadjuvant gemcitabine combination radiation therapy in the treatment of biliary tract cancer and to investigate the pathological effects of chemoradiation therapy and its impact on survival.
Methods
Chemoradiation therapy entailed three cycles of full dose of gemcitabine (1000 mg/m2 at days 1, 8, and 15, every 4 weeks) with 50–60 Gy radiation (2 Gy/day) at the main tumor and the regional and para-aortic lymph nodes. The present study included 25 patients.
Results
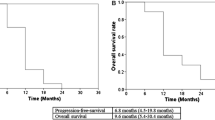
All of the patients were pathologically diagnosed before treatment. The relative dose intensity of gemcitabine was 84 %. The average dose of radiation was 53.8 Gy. Sixty percent of the patients underwent pancreatoduodenectomy, and 32 % underwent hemi-hepatectomy due to bile duct cancer (n = 24) or gall bladder cancer (n = 1). During neoadjuvant therapy, 21 patients (84 %) suffered from adverse events. The common hematological adverse events were leukopenia (44 %) and thrombocytopenia (32 %). It was necessary to exchange the plastic biliary stent in 11 patients (44 %). An R0 resection was achieved in 96 % of the patients, with pathological lymph node metastasis noted in 16 %. Moderate or marked histological changes were noted in 32 % of the patients. The 3-year overall survival rate after the first treatment was 74.6 %, with a 3.2-year observation period.
Conclusions
Neoadjuvant therapy was feasible and is expected to improve survival by controlling regional extension.




Similar content being viewed by others
Abbreviations
- BTC:
-
Biliary tract cancer
- CA19-9:
-
Carbohydrate antigen 19-9
- CEA:
-
Carcinoembryonic antigen
- CTCAE:
-
Common toxicity criteria adverse events
- OS:
-
Overall survival
- PVE:
-
Portal vein embolization
- RFS:
-
Recurrence-free survival
- UICC:
-
International Union Against Cancer
- ULN:
-
Upper limit of normal
References
Yamanaka K, Hatano E, Kanai M, Tanaka S, Yamamoto K, Narita M, Nagata H, Ishii T, Machimoto T, Taura K, Uemoto S (2014) A single-center analysis of the survival benefits of adjuvant gemcitabine chemotherapy for biliary tract cancer. Int J Clin Oncol 19:485–489
Kobayashi S, Nagano H, Marubashi S, Wada H, Eguchi H, Takeda Y, Tanemura M, Umeshita K, Doki Y, Mori M (2011) Treatment of borderline cases for curative resection of biliary tract cancer. J Surg Oncol 104:499–503
Miyakawa S, Ishihara S, Horiguchi A, Takada T, Miyazaki M, Nagakawa T (2009) Biliary tract cancer treatment: 5584 results from the Biliary Tract Cancer Statistics Registry from 1998 to 2004 in Japan. J Hepatobiliary Pancreat Surg 16:1–7
Hasegawa S, Ikai I, Fujii H, Hatano E, Shimahara Y (2007) Surgical resection of hilar cholangiocarcinoma. Analysis of survival and postoperative complications. World J Surg 31:1256–1263
Kobayashi S, Nagano H, Marubashi S, Takeda Y, Tanemura M, Konishi K, Yoshioka Y, Inoue T, Doki Y, Mori M (2009) Impact of postoperative irradiation after non-curative resection of hilar biliary cancer. J Surg Oncol 100:657–662
Kobayashi S, Miyamoto A, Shimizu J, Kashiwazaki M, Takeda Y, Ueshima S, Kim Y, Kitagawa T, Dono K, Mori M, Doki Y, Nagano H (2011) Comparison of 4-weekly vs 3-weekly gemcitabine as adjuvant chemotherapy following curative resection for biliary tract cancer: a prospective randomized controlled trial. J Cancer Ther 2:703–709
Kobayashi S, Nagano H, Sakai D, Eguchi H, Hatano E, Kanai M, Seo S, Taura K, Fujiwara Y, Ajiki T, Takemura S, Kubo S, Yanagimoto H, Toyokawa H, Tsuji A, Terajima H, Morita S, Ioka T (2014) Phase I study of adjuvant gemcitabine or S-1 in patients with biliary tract cancers undergoing major hepatectomy: KHBO1003 study. Cancer Chemother Pharmacol 74:699–709
Glazer ES, Liu P, Abdalla EK, Vauthey JN, Curley SA (2012) Neither neoadjuvant nor adjuvant therapy increases survival after biliary tract cancer resection with wide negative margins. J Gastrointest Surg 16:1666–1671
Katayose Y, Rikiyama T, Motoi F, Yamamoto K, Yoshida H, Morikawa T, Hayashi H, Kanno A, Hirota M, Satoh K, Ariga H, Suzuki M, Ohyauchi M, Kondo Y, Ikeya S, Ogawa Y, Shimosegawa T, Egawa S, Unno M (2011) Phase I trial of neoadjuvant chemoradiation with gemcitabine and surgical resection for cholangiocarcinoma patients (NACRAC study). Hepatogastroenterology 58:1866–1872
McMasters KM, Tuttle TM, Leach SD, Rich T, Cleary KR, Evans DB, Curley SA (1997) Neoadjuvant chemoradiation for extrahepatic cholangiocarcinoma. Am J Surg 174:605–608
Murad SD, Kim WR, Harnois DM, Douglas DD, Burton J, Kulik LM, Botha JF, Mezrich JD, Chapman WC, Schwartz JJ, Hong JC, Emond JC, Jeon H, Rosen CB, Gores GJ, Heimbach JK (2012) Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology 143:88–98
Takahashi H, Ohigashi H, Gotoh K, Marubashi S, Yamada T, Murata M, Ioka T, Uehara H, Yano M, Ishikawa O (2013) Preoperative gemcitabine-based chemoradiation therapy for resectable and borderline resectable pancreatic cancer. Ann Surg 258:1040–1050
Ohigashi H, Ishikawa O, Eguchi H, Takahashi H, Gotoh K, Yamada T, Yano M, Nakaizumi A, Uehara H, Tomita Y, Nishiyama K (2009) Feasibility and efficacy of combination therapy with preoperative full-dose gemcitabine, concurrent three-dimensional conformal radiation, surgery, and postoperative liver perfusion chemotherapy for T3-pancreatic cancer. Ann Surg 250:88–95
Kobayashi S, Nagano H, Marubashi S, Wada H, Eguchi H, Takeda Y, Tanemura M, Doki Y, Mori M (2010) Multi-detector computed tomography for preoperative prediction of postsurgical prognosis of patients with extrahepatic biliary cancer. J Surg Oncol 101:376–383
Kobayashi S, Nagano H, Hoshino H, Wada H, Marubashi S, Eguchi H, Takeda Y, Tanemura M, Kim T, Shimosegawa E, Hatazawa J, Doki Y, Mori M (2011) Diagnostic value of FDG-PET for lymph node metastasis and outcome of surgery for biliary cancer. J Surg Oncol 103:223–229
Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunvén P, Yamazaki S, Hasegawa H, Ozaki H (1990) Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery 107:521–527
Nagino M, Nimura Y, Kamiya J, Kondo S, Uesaka K, Kin Y, Hayakawa N, Yamamoto H (1995) Changes in hepatic lobe volume in biliary tract cancer patients after right portal vein embolization. Hepatology 21:434–439
Sobin LH, Gospodarowicz M, Wittekind C (2010) International union against cancer, TNM classification of malignant tumors, 7th edn. Wiley, New York
Shimosato Y, Oboshi S, Baba K (1971) Histological evaluation of effects of radiotherapy and chemotherapy for carcinomas. Jpn J Clin Oncol 1:19–35
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M, International Study Group on Pancreatic Fistula Definition (2005) Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 138(1):8–13
Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C, Banting S, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Yokoyama Y, Fan ST, Nimura Y, Figueras J, Capussotti L, Büchler MW, Weitz J (2011) Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 149(5):713–724
Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J, ABC-02 Trial Investigators (2010) Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362:1273–1281
Nakashima S, Kobayashi S, Sakai D, Tomokuni A, Tomimaru Y, Hama N, Wada H, Kawamoto K, Marubashi S, Eguchi H, Matsuura N, Doki Y, Mori M, Nagano H (2014) Prognostic impact of tumoral and/or peri-tumoral stromal SPARC expressions after surgery in patients with biliary tract cancer. J Surg Oncol 110:1016–1022
Aoba T, Ebata T, Yokoyama Y, Igami T, Sugawara G, Takahashi Y, Nimura Y, Nagino M (2013) Assessment of nodal status for perihilar cholangiocarcinoma: location, number, or ratio of involved nodes. Ann Surg 257:718–725
Yamada D, Kobayashi S, Wada H, Kawamoto K, Marubashi S, Eguchi H, Ishii H, Nagano H, Doki Y, Mori M (2013) Role of crosstalk between interleukin-6 and transforming growth factor-beta 1 in epithelial-mesenchymal transition and chemoresistance in biliary tract cancer. Eur J Cancer 49:1725–1740
Nakashima S, Kobayashi S, Nagano H, Tomokuni A, Tomimaru Y, Asaoka T, Hama N, Wada H, Kawamoto K, Marubashi S, Eguchi H, Doki Y, Mori M (2015) BRCA/Fanconi anemia pathway implicates chemoresistance to gemcitabine in biliary tract cancer. Cancer Sci 106:584–591
Authors’ contributions
Shogo Kobayashi, Kunihito Gotoh, Terumasa Yamada, Hiroaki Ohigashi, Osamu Ishikawa, and Kinji Nishiyama involved in the study conception and design. Shogo Kobayashi, Kunihito Gotoh, Hidenori Takahashi, Hirofumi Akita, Shigeru Marubashi, Terumasa Yamada, Teruki Teshima, and Kinji Nishiyama involved in data acquisition. Shogo Kobayashi, Akira Tomokuni, Kunihito Gotoh, Hidenori Takahashi, Hirofumi Akita, Masahiko Yano, Hiroaki Ohigashi, Osamu Ishikawa, and Masato Sakon involved in data analysis and interpretation. Shogo Kobayashi drafted the manuscript. Teruki Teshima, Hiroaki Ohigashi, Osamu Ishikawa, and Masato Sakon critically revised the paper.
Funding
This study was funded by a Grant-in-Aid for Scientific Research (C, 15K10202).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional review board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Kobayashi, S., Tomokuni, A., Gotoh, K. et al. Evaluation of the safety and pathological effects of neoadjuvant full-dose gemcitabine combination radiation therapy in patients with biliary tract cancer. Cancer Chemother Pharmacol 76, 1191–1198 (2015). https://doi.org/10.1007/s00280-015-2908-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2908-3