Abstract
Background
Diarrhea is a common toxicity of chemotherapy, but the practice of reporting only severe grades (≥ 3) in clinical trials results in misleading conclusions of significance. Epidemiology remains poorly described, and effects of multi-cycle regimens have not been investigated. To better understand the risks, symptom burden and consequences of CID, we studied patients receiving chemotherapy for colorectal cancer (CRC).
Methods
One hundred and fourteen patients receiving FOLFOX (95 patients, 530 cycles), FOLFOX + monoclonal antibodies (10 patients, 49 cycles) or FOLFIRI (9 patients, 50 cycles) were enrolled. CID was identified from diaries at baseline and daily during up to 8 chemotherapy cycles using supplemental questions on the Oral Mucositis Daily Questionnaire, a valid tool for collecting patient-reported outcomes of regimen-related mucosal injury. Patients scored CID severity from 0 “none” to 10 “worst possible,” and quantity from “little” to “severe” on a 5-point scale. Quality of life was measured using the FACT-G, and fatigue using the FACIT fatigue scale.
Results
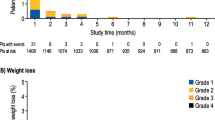
CID occurred in 89 % of patients on FOLFIRI, 50 % on FOLFOX + monoclonal antibodies and 56 % on FOLFOX alone. The risk of a first episode was highest during Cycle 1 (35 %) and dropped to <10 % during Cycles 3–5. Patients with CID reported poorer quality of life scores than those without CID (77.1 vs 80.7).
Conclusions
Diarrhea occurs more commonly than typically appreciated during chemotherapy for CRC. Risk is highest during first exposure, suggesting variable susceptibility. Identification of this high-risk subgroup for prophylaxis could improve the quality of life.



Similar content being viewed by others
References
Stein A (2010) Chemotherapy-induced diarrhea: pathophysiology, frequency and guideline-based management. Ther Adv Med Oncol 2(1):51–63
Sonis ST, Elting LS, Keefe D, Peterson DE, Schubert M, Hauer-Jensen M, NebiyouBekele B, Raber-Durlacher J, Donnelly PJ, Rubenstein EB (2004) Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 100(9):1995–2025
Gibson RJ, Keefe DM, Lalla RV et al (2013) Systematic review of agents for the management of gastrointestinal mucositis in cancer patients. Support Care Cancer 21(1):313–326. doi:10.1007/s00520-012-1644-z (Epub 2012 Nov 10)
Elting LS, Keefe DM, Sonis ST et al (2008) Patient-reported measurements of oral mucositis in head and neck cancer patients treated with radiotherapy with or without chemotherapy: demonstration of increased frequency, severity, resistance to palliation, and impact on quality of life. Cancer 113(10):2704–2713
Stiff PJ, Erder H, Bensinger WI et al (2006) Reliability and validity of a patient self-administered daily questionnaire to assess impact of oral mucositis (OM) on pain and daily functioning in patients undergoing autologous hematopoietic stem cell transplantation (HSCT). Bone Marrow Transplant 37(4):393–401
Brucker P, Yost K, Cashy J, Webster K, Cella D (2005) General population and cancer patient norms for the functional assessment of cancer therapy—General (FACT-G). Eval Health Prof 28(2):192–211
Butt Z, Lai JS, Rao D, Heinemann AW, Bill A, Cella D (2013) Measurement of fatigue in cancer, stroke, and HIV using the functional assessment of chronic illness therapy—fatigue (FACIT-F) scale. J Psychosom Res 74(1):64–68. doi:10.1016/j.jpsychores.2012.10.011 (Epub 2012 Nov)
Cella D, Hahn EA, Dineen K (2002) Meaningful change in cancer-specific quality of life scores: differences between improvement and worsening. Qual Life Res 11:207–221
Sharif S, O’Connell MJ, Yothers G, Lopa S, Wolmark N (2008) FOLFOX and FLOX regimens for the adjuvant treatment of resected stage II and III colon cancer. Cancer Invest 26(9):956–963
Acknowledgments
This study was funded by unrestricted grants from Amgen and from Helsinn Healthcare made to the Triad Burden of Illness Study Research Group.
Conflict of interest
Drs. Aprile, Barsevick, Bonaventura, Elting, Koczwara, Nguyen and Selva-Nayagam have no additional conflicts to declare. Dr. Keefe has received research funding from Entera Healthcare, Merck and Helsinn, and has a consultancy agreement with Pfizer. She is an advisor to Soligenix. Dr. Sonis is an employee of Biomodels, is a consultant at Clinical Assistance Programs, BioAlliance, Izun, Piramel, Inform Genomics (founder), Pfizer, Access, Novartis, Merck and Supportive Therapeutics. He is an advisor to Avaxia, Galera, Polymedix, Synedgen, Soligenix, Pfizer and Reata. Dr. Sonis reports Advisory roles with Biomodels, LLC, Actogenix, Avaxia, BioAlliance, Galera, Izun, Polymedix, Piramal, Synedgen, Soligenix, Pfizer, Reata, Access, Novartis and Merck. He reports personal fees from Clinical Assistance Programs and Inform Genomics; in addition, Dr. Sonis has a patent US Patent 6,458,777 issued, a patent US Patent 6,663,850 issued, a patent US Patent 6,713,463 issued, a patent US Patent 6,841,578 B2 issued and a patent US Patent 7,297,123 issued.
Author information
Authors and Affiliations
Corresponding author
Additional information
Steven M. Grunberg: Deceased.
Rights and permissions
About this article
Cite this article
Keefe, D.M., Elting, L.S., Nguyen, H.T. et al. Risk and outcomes of chemotherapy-induced diarrhea (CID) among patients with colorectal cancer receiving multi-cycle chemotherapy. Cancer Chemother Pharmacol 74, 675–680 (2014). https://doi.org/10.1007/s00280-014-2526-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2526-5