Abstract
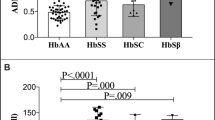
Vascular complications of sickle cell anemia (SCA) are influenced by many factors. Elevated plasma homocysteine (Hcy) is supposed to be an independent risk factor and is either genetic or nutritional origin. The present study evaluated the plasma Hcy level, MTHFR C677T gene polymorphism, effect of folic acid (FA) supplementation‚ and hemato-biochemical parameters in SCA and their effect on the vaso-occlusive crisis (VOC) in SCA patients of an Asian-Indian haplotype population. One hundred twenty cases of SCA (HbSS) and 50 controls with normal hemoglobin(HbAA) were studied. It was found that the plasma Hcy level is significantly higher (p < 0.0001) in patients with SCA (22.41 ± 7.8 μmol/L) compared to controls (13.2 ± 4.4 μmol/L). Moreover, patients without FA supplementation had a significantly (p < 0.001) higher Hcy level (27 ± 7 μmol/L) compared to those with supplementation (17.75 ± 5.7 μmol/L). Turkey-Kramer multiple comparison tests show that there is a significant difference (p < 0.05) in HbF percent, hemoglobin (Hb), platelet count, serum bilirubin (direct:Bil-D and total:Bil-T), aspartate transaminase (AST), lactate dehydrogenase (LDH), and plasma Hcy levels between mild and severe VOC. Between moderate VOC and severe VOC, there was a significant difference (p < 0.05) in HbF%, Bil-D, AST, Hcy. Pearson correlation revealed that plasma Hcy had a significantly (p < 0.05) positive correlation with AST, serum bilirubin (indirect and total), LDH, jaundice, stroke, VOC per year, and hospitalization per year whereas it was inversely correlated with HbF percentage, Hb level, and FA treatment. In the study population, increased plasma Hcy level, hemolysis, and platelet activation were found to influence VOC in SCA.

Similar content being viewed by others
References
Ingram VM (1956) A specific chemical difference between the globins of normal human and sickle-cell anaemia haemoglobin. Nature. 178(4537):792–794
Eaton WA, Hofrichter J (1987) Hemoglobin S gelation and sickle cell disease. Blood. 70(5):1245–1266
Kato GJ, Piel FB, Reid CD, Gaston MH, Ohene-Frempong K, Krishnamurti L et al (2018) Sickle cell disease. Nat Rev Dis Primers 4:18010. https://doi.org/10.1038/nrdp.2018.10
Mohan IV, Jagroop IA, Mikhailidis DP, Stansby GP (2008) Homocysteine activates platelets in vitro. Clin Appl Thromb Hemost 14(1):8–18. https://doi.org/10.1177/1076029607308390
Stuart MJ, Nagel RL (2004) Sickle-cell disease. Lancet 364(9442):1343–1360. https://doi.org/10.1016/S0140-6736(04)17192-4
Kar BC (1991) Sickle cell disease in India. J Assoc Physicians India 39(12):954–960
Kato GJ, Steinberg MH, Gladwin MT (2017) Intravascular hemolysis and the pathophysiology of sickle cell disease. J Clin Invest 127(3):750–760. https://doi.org/10.1172/JCI89741
Wilcken DE, Wilcken B et al (1976) J Clin Invest 57(4):1079–1082. https://doi.org/10.1172/JCI108350
Carey MC, Fennelly JJ, FitzGerald O, Homocystinuria II (1968) Homocystinuria. II. Subnormal serum folate levels increased folate clearance and effects of folic acid therapy. Am J Med 45(1):26–31. Available from: http://www.ncbi.nlm.nih.gov/pubmed/5658866. https://doi.org/10.1016/0002-9343(68)90004-1
Kang SS, Wong PW, Norusis M (1987) Homocysteinemia due to folate deficiency. Metabolism 36(5):458–462. https://doi.org/10.1016/0026-0495(87)90043-6
Pandey S, Pandey HR, Mishra RM, Pandey S, Saxena R (2012) Increased homocysteine level in Indian sickle cell anemia patients. Indian J Clin Biochem 27(1):103–104. https://doi.org/10.1007/s12291-011-0158-7
Abby SL, Harris IM, Harris KM (1998) Homocysteine and cardiovascular disease. J Am Board Fam Pract 11(5):391–398. https://doi.org/10.3122/15572625-11-5-391
Sati’Abbas S, Abul–Razak N, Mustafa N, Ali RA (2011) Homocysteine, folic acid, vitamin B12 and pyridoxine: effects on vaso-occlusive crisis in sickle cell anemia and sickle–thalassemia. Iraqi Acad Sci J 10(4):473–479 https://www.iasj.net/iasj?func=article&aId=43573
Luo F, Liu X, Wang S, Chen H (2006) Effect of homocysteine on platelet activation induced by collagen. Nutrition 22(1):69–75. https://doi.org/10.1016/j.nut.2005.04.012
Undas A, Stepień E, Plicner D, Zielinski L, Tracz W (2007) Elevated total homocysteine is associated with increased platelet activation at the site of microvascular injury: effects of folic acid administration. J Thromb Haemost 5(5):1070–1072. https://doi.org/10.1111/j.1538-7836.2007.02459.x
Lowenthal EA, Mayo MS, Cornwell PE, Thornley-Brown D (2000) Homocysteine elevation in sickle cell disease. J Am Coll Nutr 19(5):608–612. https://doi.org/10.1080/07315724.2000.10718958
Raouf AA, Hamdy MM, Badr AM, Shalaan O, Sakr M, Rahman AR (2018) Effect of homocysteine and folic acid on vaso-occlusive crisis in children with sickle cell disease. Egypt J Haematol 43(3):115–118
Graham IM, Daly LE, Refsum HM, Robinson K, Brattstr LE, Ueland PM et al (1997) Plasma homocysteine for vascular disease. JAMA 277(22):1775–1781. https://doi.org/10.1001/jama.1997.03540460039030
Guthikonda S, Haynes WG (2006) Homocysteine: role and implications in atherosclerosis. Curr Atheroscler Rep 8(2):100–106. https://doi.org/10.1007/s11883-006-0046-4
Ueland PM, Hustad S, Schneede J, Refsum H, Vollset SE (2001) Biological and clinical implications of the MTHFR C677T polymorphism. Trends Pharmacol Sci 22(4):195–201. Available from: http://tips.trends.com. https://doi.org/10.1016/S0165-6147(00)01675-8
Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG et al (1995) A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 10(1):111–113. https://doi.org/10.1038/ng0595-111
Jit BP, Mohanty PK, Purohit P, Das K, Patel S, Meher S, Mohanty JR, Sinha S, Behera RK, Das P (2019) Association of fetal hemoglobin level with frequency of acute pain episodes in sickle cell disease (HbS-only phenotype) patients. Blood Cells Mol Dis 75:30–34
Mashon RS, Dash PM, Khalkho J, Dash L, Mohanty PK, Patel S, Mohanty RC, Das BS, Das UK, Das PK, Patel DK (2009) Higher fetal hemoglobin concentration in patients with sickle cell disease in eastern India reduces frequency of painful crisis. Eur J Haematol 83(4):383–384
Steinberg MH (2005) Predicting clinical severity in sickle cell anaemia. Br J Haematol 129(4):465–481
Ohene-Frempong K, Weiner SJ, Sleeper LA, Miller ST, Embury S, Moohr JW et al (1998) Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 91(1):288–294
Platt OS, Thorington BD, Brambilla DJ, Milner PF, Rosse WF, Vichinsky E, Kinney TR (1991) Pain in sickle cell disease: rates and risk factors. N Engl J Med 325(1):11–16
Bailey K, Morris JS, Thomas P, Serjeant GR (1992) Fetal haemoglobin and early manifestations of homozygous sickle cell disease. Arch Dis Child 67(4):517–520
Piel FB, Patil AP, Howes RE, Nyangiri OA, Gething PW, Dewi M et al (2013) Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet 381(9861):142–151. https://doi.org/10.1016/S0140-6736(12)61229-X
Hockham C, Bhatt S, Colah R, Mukherjee MB, Penman BS, Gupta S et al (2018) The spatial epidemiology of sickle-cell anaemia in India. Sci Rep 8(1):17685. https://doi.org/10.1038/s41598-018-36077-w
Mohanty D, Mukherjee MB (2002) Sickle cell disease in India. Curr Opin Hematol 9(2):117–122
Patel DK, Mashon RS, Patel S (2010) Epidemiology and clinical aspects of sickle cell disease in India. Orissa Phys J 6:19–23
Charache S, Terrin ML, Moore RD, Dover GJ, McMahon RP, Barton FB et al (1995) Investigators of the multicenter study of hydroxyurea. Design of the multicenter study of hydroxyurea in sickle cell anemia. Control Clin Trials 16(6):432–446. https://doi.org/10.1016/S0197-2456(95)00098-4
Reid C, Davies A (2004) The World Health Organization three-step analgesic ladder comes of age. Palliat Med 18(3):175–176. https://doi.org/10.1191/0269216304pm897ed
Rees DC, Olujohungbe AD, Parker NE, Stephens AD, Telfer P, Wright J (2003) Guidelines for the management of the acute painful crisis in sickle cell disease. Br J Haematol 120(5):744–752
Ballas SK, Bauserman RL, McCarthy WF, Castro OL, Smith WR, Waclawiw MA (2010) Investigators of the multicenter study of hydroxyurea in sickle cell anemia. Hydroxyurea and acute painful crises in sickle cell anemia: effects on hospital length of stay and opioid utilization during hospitalization, outpatient acute care contacts, and at home. J Pain Symptom Manag 40(6):870–882
Kang SS, Wong PW, Malinow MR (1992) Hyperhomocyst(e)inemia as a risk factor for occlusive vascular disease. Annu Rev Nutr 12(1):279–298. https://doi.org/10.1146/annurev.nu.12.070192.001431
Refsum H, Smith AD, Ueland PM, Nexo E, Clarke R, McPartlin J et al (2004) Facts and recommendations about total homocysteine determinations: an expert opinion. Clin Chem 50(1):3–2
Old JM, Higgs DR (1983) Gene analysis. Methods in haematology. In: Weatherall DJ (ed) The Thalassemias. Churchill & Livingstone, Edinburgh, p 74
Solé X, Guinó E, Valls J, Iniesta R, Moreno V (2006) SNPStats: a web tool for the analysis of association studies. Bioinformatics 22(15):1928–1929. https://doi.org/10.1093/bioinformatics/btl268
Serjeant GR (2001) The emerging understanding of sickle cell disease. Br J Haematol 112(1):3–18. https://doi.org/10.1046/j.1365-2141.2001.02557.x
Phillips F (2005) Vegetarian nutrition. Nutr Bull 30(2):132–167. https://doi.org/10.1111/j.1467-3010.2005.00467.x
Brattström LE, Israelsson B, Jeppsson JO, Hultberg BL (1988) Folic acid—an innocuous means to reduce plasma homocysteine. Scand J Clin Lab Invest 48(3):215–221. https://doi.org/10.3109/00365518809167487
Lin N, Qin S, Luo S, Cui S, Huang G, Zhang X (2014) Homocysteine induces cytotoxicity and proliferation inhibition in neural stem cells via DNA methylation in vitro. FEBS J 281(8):2088–2096. https://doi.org/10.1111/febs.12764
García-Morin M, López-Sangüos C, Vázquez P, Alvárez T, Marañón R, Huerta J, Cela E (2016) Lactate dehydrogenase: a marker of the severity of vaso-occlusive crisis in children with sickle cell disease presenting at the emergency department. Hemoglobin. 40(6):388–391
Najim OA, Hassan MK (2011) Lactate dehydrogenase and severity of pain in children with sickle cell disease. Acta Haematol 126(3):157–162
Neely CL, Wajima T, Kraus AP, Diggs LW, Barreras L (1969) Lactic acid dehydrogenase activity and plasma hemoglobin elevations in sickle cell disease. Am J Clin Pathol 52(2):167–169
Stojanovic KS, Steichen O, Lefevre G, Bachmeyer C, Avellino V, Grateau G et al (2012) High lactate dehydrogenase levels at admission for painful vaso-occlusive crisis is associated with severe outcome in adult SCA patients. Clin Biochem 45(18):1578–1582
Ojuawo A, Adedoyin MA, Fagbule D (1994) Hepatic function tests in children with sickle cell anaemia during vaso occlusive crisis. Cent Afr J Med 40(12):342–345
Durand P, Lussier-Cacan S, Blache D (1997) Acute methionine load-induced hyperhomocysteinemia enhances platelet aggregation, thromboxane biosynthesis, and macrophage-derived tissue factor activity in rats. FASEB J 11(13):1157–1168
Westwick J, Watson-Williams EJ, Krishnamurthi S, Marks G, Ellis V, Scully MF et al (1983) Platelet activation during steady state sickle cell disease. J Med 14(1):17–36
Gardner K, Thein SL (2015) Super-elevated LDH and thrombocytopenia are markers of a severe subtype of vaso-occlusive crisis in sickle cell disease. Am J Hematol 90(10):E206–E207
Curtis SA, Danda N, Etzion Z, Cohen HW, Billett HH (2015) Elevated steady state WBC and platelet counts are associated with frequent emergency room use in adults with sickle cell anemia. PLoS One 10(8):e0133116. https://doi.org/10.1371/journal.pone.0133116
Brzoska T, Kato GJ, Sundd P (2019) The role of platelets in sickle cell disease. InPlatelets, 4th edn, pp 563–580. https://doi.org/10.1016/B978-0-12-813456-6.00031-X
Alhandalous CH, Han J, Hsu L, Gowhari M, Hassan J, Molokie R, Abbasi TA, Gordeuk VR (2015) Platelets decline during Vasoocclusive crisis as a predictor of acute chest syndrome in sickle cell disease. Am J Hematol 90(12):E228–E229
Cronin S, Furie KL, Kelly PJ (2005) Dose-related association of MTHFR 677T allele with risk of ischemic stroke: evidence from a cumulative meta-analysis. Stroke 36(7):1581–1587. https://doi.org/10.1161/01.STR.0000169946.31639.af
Acknowledgments
We thank Prof. Manoj Kumar Mohapatra (Dean & Principal) and Director, V.S.S. Institute of Medical Sciences & Research, Burla, Sambalpur, Odisha, for permitting us to carry out this study. SM expresses his gratitude to the Hon’ble Vice Chancellor, Fakir Mohan University, for supporting the research work.
Funding
The Odisha Sickle Cell Project, National Health Mission, Odisha, provided funding support.
Author information
Authors and Affiliations
Contributions
Conceptualization and design: SM, BPD, and PKM. Data collection: SM, SP, KD, SD, BPJ, MMM. Laboratory work: SM. Data analysis and interpretation: SM, SP, PD, BPD, PKM. Manuscript writing: all authors. Final approval of manuscript: all authors.
Corresponding authors
Ethics declarations
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional ethical committee of VIMSAR, Burla, Odisha (VIREC-No.2016/I-F-CT-01/008) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed written consent was obtained from all individual participants included in the study.
Financial relationship
The authors declare that they have no financial relationship with the organization that sponsored for the research work. The funding agencies had no involvement in study design, data collection, data analysis, and data interpretation.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Table 4
Alleles and genotype frequency (%) of MTHFR C677T in SCA and control (DOCX 11 kb)
Supplementary Table 5
Association between the clinical presentation of SCA and MTHFR C677T genotype (DOCX 12 kb)
Supplementary Figure 1
Mutation detection by gel documentation for MTHFR (677 C > T) (PNG 1037 kb)
Rights and permissions
About this article
Cite this article
Meher, S., Patel, S., Das, K. et al. Association of plasma homocysteine level with vaso-occlusive crisis in sickle cell anemia patients of Odisha, India. Ann Hematol 98, 2257–2265 (2019). https://doi.org/10.1007/s00277-019-03776-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-019-03776-x