Abstract
Childhood leukaemia survivors (CLS) are known to have developed long-term impairment of lung function. The reasons for that complication are only partially known. The aims of this study were to assess pulmonary function in CLS and identify (1) risk factors and (2) clinical manifestations for the impairment of airflow and lung diffusion. The study group included 74 CLS: 46 treated with chemotherapy alone (HSCT−), 28 with chemotherapy and haematopoietic stem cell transplantation (HSCT+), and 84 healthy subjects (control group (CG)). Spirometry and diffusion limit of carbon monoxide (DLCO) tests were performed in all subjects. Ten (14%) survivors had restrictive, five (7%) had obstructive pattern, and 47 (66%) had reduced DLCO. The age at diagnosis, type of transplant, and type of conditioning regimen did not significantly affect the pulmonary function tests. The DLCO%pv were lower in CLS than in CG (p < 0.03) and in the HSCT+ than in the HSCT− survivors (p < 0.05). The pulmonary infection increased the risk of diffusion impairment (OR 5.1, CI 1.16–22.9, p = 0.019). DLCO was reduced in survivors who experienced CMV lung infection (p < 0.001). The main symptom of impaired lung diffusion was poor tolerance of exercise (p < 0.005). The lower lung diffusion capacity is the most frequent abnormality in CLS. HSCT and pulmonary infection, in particular with CMV infection, are strong risk factors for impairment of lung diffusion capacity in CLS. Clinical manifestation of DLCO impairment is poor exercise tolerance. A screening for respiratory abnormalities in CLS seems to be of significant importance.
Similar content being viewed by others
Introduction
The survival rate of patients with childhood neoplasm has considerably improved in the last decade [1]. Currently, the number of childhood cancer survivors in Europe ranges between 300,000 and 500,000 [2]. However, survivors are known to be at risk of serious vital organ system toxicities and long-term side effects associated with anti-neoplastic therapy which influence their quality of life [3,4,5]. An American multi-institutional study reported that over 60% of cancer survivors after oncology therapy in childhood had at least one, and 20% had multiple chronic health conditions [4, 6]. Observations from Europe are similar and show that more than 50% of the survivors present at least one side effect, with a growing prevalence and severity with time after the completion of oncology treatment [2, 7,8,9,10]. The nationwide study performed in the largest cohort of Polish childhood cancer survivors showed that over 80% of them had one or more symptoms of organ dysfunction [11].
Haematologic malignancies are the most frequent type of childhood neoplasms with the highest prevalence of acute lymphoblastic leukaemia (ALL; 70–80% of patients) and acute non-lymphoblastic leukaemia (ANLL; approximately 10–20% of patients). The treatment of choice for leukaemia is chemotherapy which can be used in conjunction with other therapies in selected cases, such as radiotherapy of the central nervous system, or haematopoietic blood steam transplantation (HSCT). The identification of the individual factors responsible for the impairment of pulmonary function in the treatment of leukaemia is difficult because of the interaction of many variables (cytostatic drugs, radiotherapy, HSCT, pulmonary infections, individual predisposition). Pulmonary toxicity after oncology treatment may become clinically evident many years later, and may adversely affect the long-term survival as well as the quality of life of cancer childhood survivors. Damage to the alveo-capillary barrier and pulmonary fibrosis are well-described as the consequences of the late toxicity in adults [12, 13]. However, much less is known about the delayed pulmonary effects of chemotherapy, and radiotherapy in children. A further important issue is whether and what is the clinical manifestation of late pulmonotoxicity after oncological treatment in childhood. The available data on risk factors for the late adverse events and clinical manifestations of pulmonary dysfunctions in long-term childhood leukaemia survivors (CLS) are conflicting [14,15,16].
The aims of this study were (1) to assess pulmonary function in leukaemic survivors after oncology treatment in childhood, (2) to identify treatment-related risk factors, and (3) to identify clinical manifestation of late occurrence impairment of air flow and lung diffusion in CLS.
Methods
Study design
In this cross-sectional observational study, 156 subjects were included. All participants and their parents (for subjects up to the age of 18) had given written informed consent. The local ethics committee accepted the study (approval no. 324/08). The study was performed in compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and was supported by local research grant no ST-554.
Patients
The inclusion criteria were (1) achievement of the remission of childhood haematologic malignance (acute lymphoblastic leukaemia (ALL) and acute non-lymphoblastic leukaemia (ANLL)), (2) a follow-up of at least 5 years after completion of therapy, (3) above 7 years of age, and (4) the ability to perform pulmonary function tests (PFT), while exclusion criteria were diagnosis of the co-existence of cardiovascular or neuromuscular disease, respiratory infection in the previous 2 weeks, thoracic deformity, neurological impairment, and the use of bronchodilators, anticholinergics in the previous 2 days. CLS were evaluated according to the study protocol by a multidisciplinary team, including a paediatric pulmonologist. The pulmonary toxicity agents during oncology treatment: the doses (g/m2/patient) of toxic cytostatic: methotrexate (MTX), cyclophosphamide (CY), cytosine arabinoside (AraC), busulfan (BU), melphalan (MEL), irradiation to lung fields (as “yes” or “no”), and type of treatment (chemotherapy alone or with HSCT) were recorded from the patient’s chart.
The number and aetiology of pneumonia were also collected. Pneumonia was defined as the occurrence of an acute pulmonary infection with radiographic signs of parenchymal shadowing, with or without physical symptoms. Aetiology of pneumonia was established based on examination of bronchial sputum and isolation of bacteria or fungi, as well as molecular (PCR) assessment of virus presence (PCR-based techniques). Further, serum levels of antibodies against Candida albicans, Aspergillus spp., and Mucormycosis for fungal and p65 antigen in the peripheral blood cells likewise PCR for cytomegalovirus (CMV) infection diagnosis were additionally performed.
A complete physical examination was performed, and clinical manifestation of the late pulmonary system dysfunction such as persistent cough (longer than 8 weeks), dyspnoea (according to the Medical Research Council (MRC) dyspnoea scale), and poor tolerance of exercise (the questionnaire for the assessment of subjective exercise tolerance) was recorded [17]. Clinical symptoms of chronic graft-versus-host disease (cGvHD) were classified according to the previously described criteria [18].
Control group
The control group (CG) was composed of 84 healthy subjects (42/42 F/M), which were recruited from healthy volunteers matched with the survivor group, aged from 7 to 25 years (mean 12.5 ± 4.5) without current and past asthma, and other chronic or congenital pulmonary diseases.
Pulmonary function tests
Spirometry and diffusion lung capacity tests were performed in all subjects using the calibrated, computerized spirometer with gas dilution system (PneumoScreen, Jaeger, Germany), according to the European Respiratory Society (ERS) and American Thoracic Society (ATS) recommendations [19] by certificated spirometry technicians. Diffusion capacity of the lung for carbon monoxide was determined using the single-breath method and corrected for haemoglobin content (DLCO) and for alveolar volume (DLCO/VA) [20]. Forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), FEV1/FVC expressed as litres (L), and a percentage of predicted normal values (%pv) were evaluated. Abnormal lung function was defined as an obstruction if FEV1 was < 80%pv and FEV1/FVC ratio reduced < 0.7, and restrictive disorder if FVC was < 80%pv and FEV1/FVC ratio ≥ 0.7 as recommended according to ATS/ERS guidelines.
Statistical analysis
Statistical analysis was performed using the software Statistica 7.1 for Windows (Stat Soft, Inc. 2016). The risk of the lung function impairment was assessed using logistic regression analysis. The Shapiro-Wilk test was used to estimate the normal or abnormal spread of analysed variables. Student T test or non-parametric tests including Mann-Whitney U, ANOVA Kruskal-Wallis, Wilcoxon test and ANOVA Friedman test, chi-square test, and correlation test (R Spearman, Pearson) were used. In all calculations, the significance level was p < 0.05.
Results
Patient characteristics
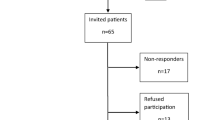
Finally, 156 subjects (74 survivors and 82 healthy subjects as CG) were included in the study (Fig. 1). CLS were treated, from 1990 to 2005, according to the protocols of the Polish Paediatric Leukaemia/Lymphoma Study Group based on the Berlin-Frankfurt-Munster group protocols, the French Society for Paediatric Oncology Group regimen LMB (lymphoma malignum type B), modified protocols for high-risk acute leukaemia New York II, and REZBFM 96 for five patients with a relapse [21,22,23,24]. All patients were Caucasian, and their characteristics are shown in Table 1.
Forty-six of CLS were treated with chemotherapy alone (HSCT−), and 28 with chemotherapy and haematopoietic stem cell transplantation (HSCT+). All CLS (n = 74) received MTX, CY, and AraC. Twenty-eight HSCT+ patients additionally received BU and MEL as a conditioning regimen. Further, 12 survivors received fractionated total body irradiation (FTBI) with a maximum dose of 9 Gy to the lungs.
Pulmonary toxicity agents are shown in Table 2. During or after oncological treatment, 40 (56%) patients had pneumonia, most often caused by fungi (no. of incidence 25). Recurrent cough was present in 19 (37%), dyspnoea in 5 (7%), and poor tolerance of exercise in 9 (13%) CLS, while no symptoms of pulmonary involvement were observed in CG. Clinical symptoms of cGvHD disease occurred in 4 survivors (gastrointestinal tract, 2; skin, 2); however, no pulmonary manifestation of GvHD was observed.
PFT
The results of PFTs are presented in Table 3. Ten survivors (14%) had a restrictive and five (7%) an obstructive (with a good response to bronchodilators) pattern. There was no significant difference in mean values of VC, FEV1, and FEV1%/VC between survivors and CG (p > 0.7). The mean values of the DLCO%pv and DLCO/VA%pv were significantly lower in CCS than in the CG (p < 0.03 and p < 0.01, respectively). Reduced DLCO%pv and DLCO/VA%pv below the 5th percentile had 47 (66%) and 17 (24%) survivors respectively.
PFT and pulmonary toxicity agents
Cumulative dose of each cytostatic drug and radiotherapy were not related to changes in spirometry or diffusion capacity test, as well the time interval to follow-up, and aged at diagnosis.
PTF and pneumonia
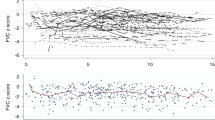
The pulmonary infection increased the risk for diffusion impairment (OR 5.1, CI 1.16–22.9, p = 0.019). Survivors who experienced CMV pneumonia (CMV+) had significantly lower DLCO%pv and DLCO/VA%pv than CG (p < 0.001; p < 0.0001) and survivors without CMV pneumonia (CMV−) (p < 0.01; p < 0.02) (Fig. 2). There were a higher number of survivors with low DLCO/VA%pv in the CMV+ than in the CMV− group (p < 0.04). DLCO%pv and DLCO/VA%pv were significantly lower also in HSCT+ survivors in comparison to both CG (p < 0.05 and p < 0.0005) and HSCT− survivors (p < 0.03 and p < 0.02, respectively) (Fig. 2). Consequently, the number of survivors with low DLCO/VA%pv was significantly higher in the HSCT+ than in the HSCT− group (p < 0.006).
The highest reduction of DLCO and DLCO/VA parameters was observed in CMV+ survivors who underwent HSCT (CMV+HSCT+): [CMV+HSCT+ vs. CMV−HSCT+ survivors (p < 0.05 p < 0.002)] and [CMV+HSCT+survivors vs. CG (p < 0.005 p < 0.00005)] (Fig. 2).
PFT and clinical manifestation
Persistent dyspnoea, cough, and poor tolerance of exercise were reported by all patients (n = 5) with obstructive abnormalities. Among all ten CLS with restriction impairment, four with HSCT+ had poor tolerance of exercise, whereas six survivors (all HSCT−) had no clinical symptoms.
Symptomatic CLS with poor tolerance of exercise had a significantly lower value of DLCO%pv and DLCO/VA%pv than asymptomatic survivors (p < 0.05, p < 0.005) (Table 4).
Discussion
The study provides insight on the late pulmonary outcomes measured by PFT in childhood leukaemia survivors at least 5 years after completion of oncology treatment (chemotherapy alone or followed by HSCT with additional conditioning megachemotherapy or total body irradiation).
The most important finding of this study is the fact that there is a prolonged difference in the pulmonary function test between survivors who had more aggressive treatment (with HSCT), as well as those who experienced pneumonia, especially of CMV origin, and both survivors without these risk factors and subjects from CG. At least 5 years after the completion of oncology therapy, deteriorated DLCO was demonstrated in most of CLS.
It is well-known that CMV pneumonia in immunocompromised patients is an acute, life-threatening illness with a high mortality rate in spite of intensive treatment with antiviral agents; however, there is little data about the long-term sequelae in such cases. Chien et al. describe seven BMT recipients, after CMV pneumonia. Five patients died, in the remaining two restrictive lung disease with lower DLCO was diagnosed [25]. Also, in kidney transplant recipients, CMV caused subclinical pulmonary dysfunction with pulmonary diffusion disturbances some months after active disease treatment [26]. In our study, children with a CMV infection had significantly lower DLCO, which was particularly expressed in the group with HSCT. In some other studies, CMV-positive leukaemic children after BMT were also found to have had lower diffusion capacity [27,28,29,30]. The mechanisms leading to the persistent damage of the alveolar-capillary barrier are not clear. Recently, a model of promoting lung damage by CMV was proposed, suggesting that CMV causes a pro-fibrotic milieu by increasing the expression CCL2 that sequesters CCR2 expressing pro-fibrotic mononuclear cells of the lung [31]. The other model was also proposed that CMV may cause an increased production and activation of TGF-β1 (transforming growth factor-β1), a multifunctional cytokine playing a key role in cell proliferation, migration, and synthesis of the extracellular matrix in the endothelium [32]. TGF-β1 may contribute to the fibrotic and vascular components of CMV pathology. Interestingly, in an animal model, TGF-β1 protein was increased in alveoli in rat lungs after radiation-induced immune suppression of CMV-infected rats [33].
Our data suggest that CMV could potentially damage the alveo-capillary barrier. However, the identification of individual factors contributing to impairment of diffusion capacity in leukemic patients is difficult because of the interaction of many different interfering variables. Nevertheless, such a strong statistical correlation between decreased diffusion capacity and CMV pneumonia as it was found in our study may suggest the direct prolonged influence of CMV infection on the pulmonary tissue.
Another finding of the present study is that the HSCT procedure affected lung diffusion. Lower diffusion values were significantly more frequent in CLS who received HSCT than in the children treated with chemotherapy alone (41% vs. 14%). Similar data on DLCO dysfunction (12–28% of patients treated with chemotherapy alone and 27–58% after HSCT) was presented in other studies [22, 27, 29, 30, 33,34,35,36,37]. These findings may be partially explained by the fact that the HSCT patients had a more intensive immunosuppressive treatment with additional radiotherapy. However, in our study, other factors, such as pulmonary infection, also affected these abnormalities.
A clinically meaningful finding is that a proportion of CLS (13%) had a poor tolerance of exercise, which is related to the decreased DCLO. Measurement of DLCO is considered a good indicator of pneumopathy—it can be lowered even if there were no clinical symptoms and the spirometry remains within normal values. In several studies with long follow-up, the pulmonary dysfunction (decreased diffusion capacity) was usually without clinical symptoms [27, 29, 30]. In contradiction to these studies, we observed an interesting relationship: survivors who complained of poorer tolerance of exercise had a significantly lower diffusion capacity (p < 0.005). This correlation was particularly strong in survivors, who underwent HSCT. One possible explanation is that an injury of the alveo-capillary barrier (reflected by lowered DLCO) in leukemic children treated with HSCT was more advanced, and therefore caused clinical symptoms. These symptoms, especially decreased ability to exercise, may reduce physical activity and, in turn, affect their quality of life and proper physical development.
Study limitations
A cross-sectional study design may suggest correlations but is limited in assigning a cause-effect relationship between CMV pneumonia and diffusion defect. Diversity of study group in terms of age and time to follow-up may have influenced the final results.
Conclusion
The results of the present study show that the lower lung diffusion capacity is the most frequent abnormality in CLS. Pulmonary infection, especially caused by CMV, is a strong risk factor for impairment of lung diffusion capacity in survivors, which results in poor exercise tolerance. The particular group of risk comprised children with additional treatment including HSCT. Since the decrease of pulmonary function and the poor tolerance of exercise occur in a proportion of survivors following treatment for childhood haematologic malignancies, a screening for respiratory abnormalities in this group seems to be of importance and be part of survivorship care plan.
Clinical implications
Childhood leukaemia survivors could be suffering from long-lasting consequence—poor tolerance of exercise, after their oncology treatment. They and their healthcare providers need more health information about this long-term risk associated with their cancer and treatment history, and to perform not only spirometry but diffusion lung capacity tests. This knowledge could improve their quality of life related to physical activity.
References
Gatta G, Botta L, Rossi S, Aareleid T, Bielska-Lasota M, Clavel J, Dimitrova N, Jakab Z, Kaatsch P, Lacour B, Mallone S, Marcos-Gragera R, Minicozzi P, Sánchez-Pérez MJ, Sant M, Santaquilani M, Stiller C, Tavilla A, Trama A, Visser O, Peris-Bonet R (2014) Childhood cancer survival in Europe 1999-2007: results of EUROCARE-5-a population-based study. Lancet Oncol 15:35–47
Winther JF, Kenborg L, Byrne J, Hjorth L, Kaatsch P, Kremer LC et al (2015) Children cancer survivor cohort in Europe. Acta Oncol 54(5):655–668. https://doi.org/10.3109/0284186X.2015.1008648
Dickerman JD (2007) The late effects of childhood cancer therapy. Pediatrics 3:119–122
Geenen MM, Cardous-Ubbink MC, Kremer LCM, van den Bos C, van der Pal HJH, Heinen RC, Jaspers MWM, Koning CCE, Oldenburger F, Langeveld NE, Hart AAM, Bakker PJM, Caron HN, van Leeuwen FE (2007) Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA 297:2705–2715
Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM et al (2006) Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 355(15):1572–1582. https://doi.org/10.1056/NEJMsa060185 25]
Phillips SM, Padgett LS, Leisenring WM, Stratton KK, Bishop K, Krull KR, Alfano CM, Gibson TM, de Moor JS, Hartigan DB, Armstrong GT, Robison LL, Rowland JH, Oeffinger KC, Mariotto AB (2015) Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiol Biomark Prev 24(4):653–666. https://doi.org/10.1158/1055-9965.EPI-14-1418
Erman N, Todorovski L, Jereb B (2012) Late somatic sequelae after treatment of childhood cancer in Slovenia. BMC Res Notes 5(1):254. https://doi.org/10.1186/1756-0500-5-254
Tantawy AA, Elbarbary N, Ahmed A, Mohamed NA, Ezz-Elarab S (2011) Pulmonary complications in survivors of childhood hematological malignancies: single-center experience. Pediatr Hematol Oncol 28:403–417
Mertens AC, Yasui Y, Liu Y, Stovall M, Hutchinson R, Ginsberg J, Sklar C, Robison LL, Childhood Cancer Survivor Study (2002) Pulmonary complications in survivors of childhood and adolescent cancer. Cancer 95:2431–2441
Mody R, Li S, Dover DC, Sallan S, Leisenring W, Oeffinger KC, Yasui Y, Robison LL, Neglia JP (2008) Twenty-five–year follow-up among survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. Blood 111:5515–5523. https://doi.org/10.1182/blood-2007-10-117150
Krawczuk-Rybak M, Panasiuk A, Stachowicz-Stencel T, Zubowska M, Skalska-Sadowska J, Sęga-Pondel D, Czajńska-Deptuła A, Sławińska D, Badowska W, Kamieńska E, Pobudejska-Pieniążek A, Wieczorek M (2018) Health status of Polish children and adolescents after cancer treatment. Eur J Pediatr 177(3):437–447. https://doi.org/10.1007/s00431-017-3066-x
Segura A, Yuste A, Ceros A, López-Tendero P, Gironés R, Pérez-Fidalgo JA, Herranz C (200) Pulmonary fibrosis induced by cyclophosphamide. Ann Pharmacother 35:894–897
Sharma S, Nadrous HF, Peters SG, Tefferi A, Litzow RM, Aubry MC, Affesa B (2005) Pulmonary complications in adult blood and marrow transplant recipients: autopsy findings. Chest. 128:1385–1392
Meyer S, Reinhard H, Gottschling S (2004) Pulmonary dysfunction in pediatric oncology patients. Pediatr Hematol Oncol 21:175–195
Ben-Abraham R, Weinbroum AA, Augerten A, Toren A, Harel R, Vardi A, Barzilay Z, Paret G (2001) Acute respiratory distress syndrome in children with malignancies- can we predicted outcome? J Crit Care 16:54–58
Horning SJ, Adhikari A, Rizk N, Hoppe RT, Olshen RA (1994) Effect of treatment for Hodgkin’s disease on pulmonary function: results of prospective study. J Clin Oncol 12:297–305
Jenney MEM, Faragher EB, Morris-Jones PH, Woodcock AA (1995) Lung function and exercise capacity in survivors childhood leukemia. Med Pediatr Oncol 24:222–230
Shulman IIM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, Hackman R, Tsoi MS, Storb R, Thomas ED (1980) Chronic graft versus host syndrome in man. Am J Med 69:204–217
Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, van der Grinten C, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J, ATS/ERS Task Force (2005) General consideration for lung function testing. Eur Respir J 26:153–161
MacIntyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten MCP et al (2005) Standardization of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 26:720–735
Radwańska U, Michalewska D, Kolecki P, Armata J, Balwierz W, Boguslawska-Jaworska J, Chybicka A, Cyklis R, Kowalczyk J, Ochocka M (1995) Standard and intermediate risk acute lymphoblastic leukemia in Poland: a report of the Polish Children’s Leukemia/Lymphoma Study Group. Acta Paediatr Jpn 37:31–36
Dłużniewska A, Balwierz W, Armata J, Balcerska A, Chybicka A, Kowalczyk J, Matysiak M, Ochocka M, Radwanska U, Rokicka-Milewska R, Sonta-Jakimczyk D, Wachowiak J, Wysocki M (2005) Twenty years of Polish experience with three consecutive protocols for treatment of childhood acute myelogenous leukemia. Leukemia. 19:2117–2124
Bogusławska-Jaworska J, Kazanowska B, Rodziewicz-Magott B, Pietras W, Armata J, Daszkiewicz P, Dłuzniewska A, Koehler M, Matysiak M, Ochocka M (1989) Results of treatment in children with B-cell lymphoma: report on the Polish Leukemia/Lymphoma Study Group. Blood Transfus 32:71–74
Wachowiak J, Labopin M, Miano M, Chybicka A, Stary J, Streba J, Masszi T, Labar B, Maschan A, Kowalczyk JR, Lange A, Holowiecki J, Kalman N, Afanassiev BV, Dini G (2008) Haematopoietic stem cell transplantation in children in eastern Europe countries 1985-2004: development, recent activity and role of the EBMT/ESH Outreach Programme. Bone Marrow Transplant 41:112–117
Chien SM, Chan CK, Kasupski G, Chamberlain D, Fyles G, Messner H (1992) Long-term sequelae after recovery from cytomegalovirus pneumonia in allogeneic bone marrow transplant recipients. Chest. 101:100–104
De Maar EF, Kas-Deelen AM, Van Der Mark TW, Tegzess AM, Ploeg RJ, van Son WJ (1999) Cytomegalovirus pneumonitis after kidney transplantation is not caused by plugging of cytomegalic endothelial cells only. Transpl Int 12:56–62
Fanfulla F, Locatelli F, Zoia MC, Bonetti GF, Spagnolatti L, Cerveri I (1997) Pulmonary complications and respiratory function changes after bone marrow transplantation in children. Eur Respir J 10:2301–2306
Quigly PM, Yeager AM, Loughlin GM (1994) The effects of bone marrow transplantation on pulmonary function in children. Pediatr Pulmonol 18:361–367
Cerveri I, Fulgoni P, Giorgani G, Zoia MC, Beccaria M, Tinelli C Locatelli F (2001) Lung function abnormalities after bone marrow transplantation in children. Has the trend recently changed? Chest. 120:1900–1906
Cerveri I, Zoia MC, Fulgoni P, Corsico A, Casali L, Tinelli C, Zecca M, Giorgiani G, Locatelli F (1999) Late pulmonary sequels after childhood bone marrow transplantation. Thorax. 54:131–135
Weight SS, Elashoff RM, Keane MP, Strieter RM, Gomperts BN, Xue YY, Ardehali A, Gregson AL, Kubak B, Fishbein MC, Saggar R, Ross DJ, Lynch JP III, Zisman DA, Belperio JA (2008) Altered levels of CC chemokines Turing pulmonary CMV predict BOS and mortality post-lung transplantation. Am J Transplant 8:1512–1522
Tabata T, Kawakatsu H, Maidji E, Sakai T, Sakai K, Fang-Hoover J, Aiba M, Sheppard D, Pereira L (2008) Induction of an epithelial integrin vβ6 in human cytomegalovirus-infected endothelial cells leads to activation of transforming growth factor-β1 and increased collagen production. American J Pathol 172:1127–1140
Haagmans BL, Teerds KJ, van den Eijnden-van Raaij AJ, Horzinek MC, Schijns VE (1997) Transforming growth factor beta production during rat cytomegalovirus infection. J Gen Virol 78:205–213
Fulgoni P, Zoia MC, Corsico A (1999) Lung function in survivors of childhood acute lymphoblastic leukemia. Chest. 116:1163–1167
Nysom K, Holm K, Olsen JH, Hertz H, Hesse B (1998) Pulmonary function after treatment for acute lymphoblastic leukemia in childhood. Br J Cancer 78:21–28
Nysom K, Holm K, Hesse B, Ulrik CS, Jacobsen N, Bisgaard H, Hertz H (1996) Lung function after allogeneic bone marrow transplantation for leukemia or lymphoma in childhood. Arch Dis Child 74:432–436
Nysom K, Holm K, Hertz H, Hesse B (1998) Risk factor for reduced pulmonary function after malignant lymphoma in childhood. Med Pediatr Oncol 30:240–248
Funding
This study was funded by the Medical University of Gdańsk, Poland (Institutional Grant ST 554).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wasilewska, E., Kuziemski, K., Niedoszytko, M. et al. Impairment of lung diffusion capacity—a new consequence in the long-term childhood leukaemia survivors. Ann Hematol 98, 2103–2110 (2019). https://doi.org/10.1007/s00277-019-03745-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-019-03745-4