Abstract
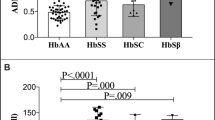
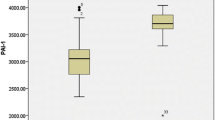
One complication of thalassemia is thromboembolism (TE), which is caused by an abnormal red blood cell surface, as well as endothelial and platelet activation. These findings are commonly observed in severe β-thalassemia. However, limited information on α-thalassemia exists. This study enrolled subjects with deletional and non-deletional α-thalassemia and normal controls (NC). Plasma and serum of subjects were tested for endothelial activation markers including thrombomodulin (TM), vascular cell adhesion molecule-1 (VCAM-1), and von Willebrand factor antigen as well as platelet activation markers including thromboxane B2 and platelet factor 4. A total of 179 subjects were enrolled: 29 in the deletional group (mean age 13.3 ± 4.4 years), 31 in the non-deletional group (mean age 12.9 ± 4.8 years), and 119 in the NC group (mean age 13.6 ± 3.0 years). Twenty nine percent of subjects in the non-deletional group received regular red blood cell transfusion and iron chelator administration. Serum ferritin level was higher in the non-deletional group than that in the deletional group. Multivariate analysis demonstrated that VCAM-1 and TM levels were increased significantly in α-thalassemia compared with NC group (816.8 ± 131.0 vs 593.9 ± 49.0 ng/ml, and 4.9 ± 0.7 vs 4.0 ± 0.4 ng/ml, P < 0.001 respectively). VCAM-1 and TM levels in the non-deletional group were significantly higher than that in the deletional group. The present study demonstrated endothelial activation in children with α-thalassemia disease, especially those in the non-deletional group, which might be one risk factor for TE in α-thalassemia disease.


Similar content being viewed by others
Abbreviations
- BMI:
-
Body mass index
- β-TG:
-
β-Thromboglobulin
- CBC:
-
Complete blood count
- CECs:
-
Circulating endothelial cells
- CS:
-
Hb constant spring
- DNA:
-
Deoxyribonucleic acid
- ELAM:
-
E-selectin
- F1.2:
-
Prothrombin fragment 1 + 2
- Hb:
-
Hemoglobin
- HPLC:
-
High-performance liquid chromatography
- ICAM-1:
-
Intercellular adhesion molecule-1
- MCH:
-
Mean corpuscular hemoglobin
- MCV:
-
Mean corpuscular volume
- NC:
-
Normal controls
- NTDT:
-
Non-transfusion dependent thalassemia
- PF4:
-
Platelet factor 4
- PS:
-
Hb Paksé
- RBC:
-
Red blood cell
- RDW:
-
Red cell distribution width
- TAT:
-
Thrombin-antithrombin complex
- TBX2:
-
Thromboxane B2
- TDT:
-
Transfusion-dependent thalassemia
- TE:
-
Thromboembolism
- TM:
-
Thrombomodulin
- VCAM-1:
-
Vascular cell adhesion molecule-1
- VEGF:
-
Vascular endothelial growth factor
- VWF:Ag:
-
Von Willebrand factor antigen
References
Fucharoen S, Winichagoon P (1992) Thalassemia in Southeast Asia: problems and strategy for prevention and control. Southeast Asian J Trop Med Public Health 23:647–655
Kohne E (2011) Hemoglobinopathies: clinical manifestations, diagnosis, and treatment. Dtsch Arztebl Int 108:532–540. https://doi.org/10.3238/arztebl.2011.0532
Kelly N (2012) Thalassemia. Pediatr Rev 33:434–435. https://doi.org/10.1542/pir.339-434
Vichinsky EP (2005) Changing patterns of thalassemia worldwide. Ann N Y Acad Sci 1054:18–24. https://doi.org/10.1196/annals.1345.003
Williams TN, Weatherall DJ (2012) World distribution, population genetics, and health burden of the hemoglobinopathies. Cold Spring Harb Perspect Med 2:1–14. https://doi.org/10.1101/cshperspect.a011692
Wasi P, Na-Nakorn S, Pootrakul S, Sookanek M, Disthasongchan P, Pornpatkul M, Pornpatkul M (1969) Alpha- and beta-thalassemia in Thailand. Ann N Y Acad Sci 165:60–82. https://doi.org/10.1111/j.1749-6632.1969.tb27777.x
Vichinsky E (2010) Complexity of alpha thalassemia: growing health problem with new approaches to screening, diagnosis, and therapy. Ann N Y Acad Sci 1202:180–187. https://doi.org/10.1111/j.1749-6632.2010.05572.x
Fucharoen S, Viprakasit V (2009) Hb H disease: clinical course and disease modifiers. Am Soc Hematol Educ Program 2009:26–34. https://doi.org/10.1182/asheducation-2009.1.26
Vichinsky EP (2013) Clinical manifestations of α-thalassemia. Cold Spring Harb Perspect Med 3:1–10. https://doi.org/10.1101/cshperspect.a011742
Laosombat V, Viprakasit V, Chotsampancharoen T, Wongchanchailert M, Khodchawan S, Chinchang W, Sattayasevana B (2009) Clinical features and molecular analysis in Thai patients with HbH disease. Ann Hematol 88:1185–1192. https://doi.org/10.1007/s00277-009-0743-5
Chui DH, Fucharoen S, Chan V (2003) Hemoglobin H disease: not necessarily a benign disorder. Blood 101:791–800. https://doi.org/10.1182/blood-2002-07-1975
Harteveld CL, Higgs DR (2010) α-Thalassaemia. Orphanet J Rare Dis 5:13. https://doi.org/10.1186/1750-1172-5-13
Sirachainan N (2013) Thalassemia and the hypercoagulable state. Thromb Res 132:637–641. https://doi.org/10.1016/j.thromres.2013.09.029
Tso SC, Chan TK, Todd D (1982) Venous thrombosis in haemoglobin H disease after splenectomy. Aust NZ J Med 12:635–638. https://doi.org/10.1111/j.1445-5994.1982.tb02655.x
Singer ST, Kim HY, Olivieri NF, Kwiatkowski JL, Coates TD, Carson S, Neufeld E, Cunningham MJ, Giardina PJ, Mueller BU, Quinn CT, Fung E, Vichinsky E, for the Thalassemia Clinical Research Network (2009) Hemoglobin H-constant spring in North America: an alpha thalassemia with frequent complications. Am J Hematol 84:759–761. https://doi.org/10.1002/ajh.21523
Sun NA, Cheng P, Deng DH, Liu RR, Lai YR (2016) Analysis of the genetic variants associated with recurrent thromboembolism in a patient with hemoglobin H disease following splenectomy: a case report. Biomed Rep 5:23–26. https://doi.org/10.3892/br.2016.674
Sonakul D, Fucharoen S (1992) Pulmonary thromboembolism in thalassemic patients. Southeast Asian J Trop Med Public Health 23:25–28
Winichakoon P, Tantiworawit A, Rattanathammethee T, Hantrakool S, Chai-Adisaksopha C, Rattarittamrong E, Norasetthada L, Charoenkwan P (2015) Prevalence and risk factors for complications in patients with nontransfusion dependent alpha-and beta-thalassemia. Anemia 2015:793025. https://doi.org/10.1155/2015/793025
Bunyaratvej A, Fucharoen S, Butthep P, Sae-ung N, Kamchonwongpaisan S, Khuhapinant A (1995) Alterations and pathology of thalassemic red cells: comparison between alpha-and beta-thalassemia. Southeast Asian J Trop Med Public Health 26:257–260
Butthep P, Bunyaratvej A, Funahara Y, Kitaguchi H, Fucharoen S, Sato S, Bhamarapravati N (1995) Alterations in vascular endothelial cell-related plasma proteins in thalassaemic patients and their correlation with clinical symptoms. Thromb Haemost 74:1045–1049. https://doi.org/10.1055/s-0038-1649879
Carlos TM, Harlan JM (1994) Leukocyte-endothelial adhesion molecules. Blood 84:2068–2101
Butthep P, Bunyaratvej A, Funahara Y, Kitaguchi H, Fucharoen S, Sato S, Bhamarapravati N (1997) Possible evidence of endothelial cell activation and disturbance in thalassemia: an in vitro study. Southeast Asian J Trop Med Public Health 28:141–148A
Butthep P, Rummavas S, Wisedpanichkij R, Jindadamrongwech S, Fucharoen S, Bunyaratvej A (2002) Increased circulating activated endothelial cells, vascular endothelial growth factor, and tumor necrosis factor in thalassemia. Am J Hematol 70:100–106. https://doi.org/10.1002/ajh.10101
Chansai S, Fucharoen S, Fucharoen G, Jetsrisuparb A, Chumpia W (2018) Elevations of thrombotic biomarkers in hemoglobin H disease. Acta Haematol 139:47–51. https://doi.org/10.1159/000486157
Sirachainan N, Chuansumrit A, Kadegasem P, Sasanakul W, Wongwerawattanakoon P, Mahaklan L, Mahaklan L (2016) Normal hemostatic parameters in children and young adults with α-thalassemia diseases. Thromb Res 146:35–42. https://doi.org/10.1016/j.thromres.2016.08.024
Taher A, Vichinsky E, Musallam K, Cappellini MD, Viprakasit V, Weatherall D, editor (2013) Guidelines for the Management of Non Transfusion Dependent Thalassaemia (NTDT) [Internet]. Thalassaemia International Federation, Nicosia. Available from http://www.ncbi.nlm.nih.gov/books/NBK190453/. Accessed 4 April 2019
Prasartkaew S, Bunyaratvej A, Fucharoen S, Wasi P (1986) Comparison of erythrocyte antioxidative enzyme activities between two types of haemoglobin H disease. J Clin Pathol 39:1299–1303. https://doi.org/10.1136/jcp.39.12.1299
Bunyaratvej A, Butthep P, Fucharoen S, Saw D (1992) Erythrocyte volume and haemoglobin concentration in haemoglobin H disease: discrimination between the two genotypes. Acta Haematol 87:1–5. https://doi.org/10.1159/000204704
Fucharoen S, Winichagoon P (2002) Thalassemia and abnormal hemoglobin. Int J Hematol 76:83–89. https://doi.org/10.1371/journal.pone.0108365
Boonsa S, Sanchaisuriya K, Fucharoen G, Wiangnon S, Jetsrisuparb A, Fucharoen S (2004) The diverse molecular basis and hematological features of Hb H and AE Bart’s diseases in Northeast Thailand. Acta Haematol 111:149–154. https://doi.org/10.1159/000076523
Bastyr EJ, Kadrofske MM, Vinik AI (1990) Platelet activity and phosphoinositide turnover increase with advancing age. Am J Med 88:601–606. https://doi.org/10.1016/00029343(90)90525-I
Trichero A, Marchetti M, Giaccherini C, Tartari CJ, Russo L, Falango A (2017) Platelet haemostatic properties in β-thalassemia: the effect of blood transfusion. Blood Transfus 15(5):413–421. https://doi.org/10.2450/2016.0033-16
Steiner M, Anastasi J, Vitamin E (1976) An inhibitor of the platelet release reaction. J Clin Invest 57(3):732–737. https://doi.org/10.1172/JCI108331
Acknowledgements
NC designed the study and reviewed the manuscript. PS performed the study and wrote the manuscript. AC, DS, and PW involved in the care of patients. PK performed the laboratory.
Funding
This work was supported by a Grant from the Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand. We thank Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval for the study protocol was obtained from the Ethical Clearance Committee on Human Rights Related to Research to Research Involving Human Subjects, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Thailand (ID 12-59-09).
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sirivadhanakul, P., Chuansumrit, A., Songdej, D. et al. Increased endothelial activation in α-thalassemia disease. Ann Hematol 98, 1593–1602 (2019). https://doi.org/10.1007/s00277-019-03672-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-019-03672-4