Abstract
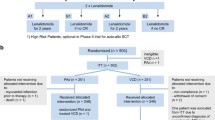
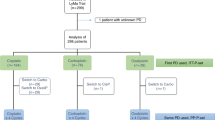
The DHAP regimen (high-dose cytarabine in combination with dexamethasone and cisplatin) with or without rituximab (DHAP+/−R) is one of the most common regimens in daily practice. It is considered the standard treatment for relapse or refractory Hodgkin’s and non-Hodgkin’s lymphoma (NHL). Cisplatin nephrotoxicity is a major concern, and other platinum compounds are being tried. We performed a monocentric retrospective analysis to evaluate the use of carboplatin, so-called DHAC+/−R regimen. The purpose was to assess the toxicity of the DHAC+/−R regimen in real-life. The Dexamethasone, Cytarabine, Carboplatin (DHAC) regimen consisted of carboplatin AUC = 5 mg/ml/min (targeted area under the curve with Calvert’s formula) on day 1, cytarabine 2 g/m2 twice a day on day 2 and IV dexamethasone 40 mg from days 1 to 4. Rituximab was administrated at 375 mg/m2 on day 1 for CD20+ NHL. The interval between courses was 21 days. During the period considered, 199 patients received DHAC+/−R. For the entire cohort, median follow-up is 24 months (range, 2–82), median OS is not reached (NR), estimated 2-year OS is 75% (95% CI, 69–83) and median progression-free survival (PFS) is 46 months (95% CI, 22-NA). Of 144 patients scheduled for autologous stem cell transplantation (ASCT), 102 (71%, NA = 2) were in response after DHAC+/−R and all except 4 underwent ASCT. Grade ≥ 3 haematological toxicities were mainly thrombocytopenia (n = 101) and anaemia (n = 95). Grade ≥ 3 neutropenia occurred in 10 patients. No grade ≥ 3 renal and one grade 3 neurological toxicity were reported. DHAC+/−R is feasible in daily practice, provides good response rates and jeopardises neither stem cell collection nor ASCT.



Similar content being viewed by others
References
Velasquez WS, Cabanillas F, Salvador P et al (1988) Effective salvage therapy for lymphoma with cisplatin in combination with high-dose Ara-C and dexamethasone (DHAP). Blood 71:117–122
Philip T, Chauvin F, Armitage J et al (1991) Parma international protocol: pilot study of DHAP followed by involved-field radiotherapy and BEAC with autologous bone marrow transplantation. Blood 77:1587–1592
Gisselbrecht C, Glass B, Mounier N et al (2010) Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol Off J Am Soc Clin Oncol 28:4184–4190. doi:10.1200/JCO.2010.28.1618
Neste EVD, Casasnovas O, André M et al (2013) Classical Hodgkin’s lymphoma: the Lymphoma Study Association guidelines for relapsed and refractory adult patients eligible for transplant. Haematologica 98:1185–1195. doi:10.3324/haematol.2012.072090
Delarue R, Haioun C, Ribrag V et al (2013) CHOP and DHAP plus rituximab followed by autologous stem cell transplantation in mantle cell lymphoma: a phase 2 study from the Groupe d’Etude des Lymphomes de l’Adulte. Blood 121:48–53. doi:10.1182/blood-2011-09-370320
Le Gouill S, Callanan M, Macintyre E (2012) Clinical, metabolic and molecular responses after 4 courses of R-DHAP and after autologous stem cell transplantation for untreated mantle cell lymphoma patients included in the LyMa Trial: a Lysa study
Hermine O, et al. (2012) Alternating courses of 3× CHOP and 3× DHAP + rituximab followed by a high dose Ara-C containing myeloablative regimen and ASCT increases overall survival when compared to 6 courses of CHOP + rituximab followed by radioimmunotherapy and ASCT in mantle cell lymphoma
Dreyling M, Thieblemont C, Gallamini A et al (2013) ESMO Consensus conferences: guidelines on malignant lymphoma. part 2: marginal zone lymphoma, mantle cell lymphoma, peripheral T-cell lymphoma. Ann Oncol 24(4):857–877. doi:10.1093/annonc/mds643
Yokoo S, Yonezawa A, Masuda S et al (2007) Differential contribution of organic cation transporters, OCT2 and MATE1, in platinum agent-induced nephrotoxicity. Biochem Pharmacol 74:477–487. doi:10.1016/j.bcp.2007.03.004
Ciarimboli G, Ludwig T, Lang D et al (2005) Cisplatin nephrotoxicity is critically mediated via the human organic cation transporter 2. Am J Pathol 167:1477–1484. doi:10.1016/S0002-9440(10)61234-5
Tixier F, Ranchon F, Iltis A et al (2016) Comparative toxicities of 3 platinum-containing chemotherapy regimens in relapsed/refractory lymphoma patients. Hematol Oncol. doi:10.1002/hon.2328
Lignon J, Sibon D, Madelaine I et al (2010) Rituximab, dexamethasone, cytarabine, and oxaliplatin (R-DHAX) is an effective and safe salvage regimen in relapsed/refractory B-cell non-Hodgkin lymphoma. Clin Lymphoma Myeloma Leuk 10:262–269. doi:10.3816/CLML.2010.n.055
Cheson BD, Horning SJ, Coiffier B et al (1999) Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol Off J Am Soc Clin Oncol 17:1244. doi:10.1200/JCO.1999.17.4.1244
Cheson BD, Pfistner B, Juweid ME et al (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25:579–586. doi:10.1200/JCO.2006.09.2403
Calvert AH, Newell DR, Gumbrell LA et al (1989) Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol 7:1748–1756
Thomas H, Boddy AV, English MW et al (2000) Prospective validation of renal function-based carboplatin dosing in children with cancer: a United Kingdom Children’s cancer study group trial. J Clin Oncol Off J Am Soc Clin Oncol 18:3614–3621
Egorin MJ, Van Echo DA, Olman EA et al (1985) Prospective validation of a pharmacologically based dosing scheme for the cis-diamminedichloroplatinum (II) analogue diamminecyclobutanedicarboxylatoplatinum. Cancer Res 45:6502–6506
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
Machover D, Delmas-Marsalet B, Misra SC et al (2010) Treatment with rituximab, dexamethasone, high-dose cytarabine, and oxaliplatin (R-DHAOx) produces a strong long-term antitumor effect in previously treated patients with follicular non-Hodgkin’s lymphoma. Biomed Pharmacother 64:83–87. doi:10.1016/j.biopha.2009.11.001
Acknowledgements
The authors acknowledge the support of the nurses of the Haematology Department of Nantes Medical University (France), the patients and their families. The data was collected from our database (Lhenabase).
BT is the recipient for a fellowship from Fondation ARC pour la Recherche contre le Cancer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
According to French legislation (articles L. 1121-1 paragraph 1 and R1121-2, Public Health Code), this observational retrospective study using non-transplanted patient data requires neither informed consent nor ethics committee approval. All transplanted patients signed an informed consent and were reported to the EBMT Registry Database.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Table 1
Dosings (g/m2). Carbo x: Carboplatin infusion at time xth, Cyta x: Cytarabine infusion at time xth, <70y: patients less than 70 years old, >=70y: patients 70years-old or older. (DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Tessoulin, B., Thomare, P., Delande, E. et al. Carboplatin instead of cisplatin in combination with dexamethasone, high-dose cytarabine with or without rituximab (DHAC+/−R) is an effective treatment with low toxicity in Hodgkin’s and non-Hodgkin’s lymphomas. Ann Hematol 96, 943–950 (2017). https://doi.org/10.1007/s00277-017-2981-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-017-2981-2