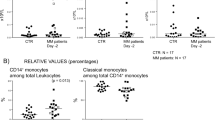
Abstract
Human leukocyte antigen DR surface expression in “classical” CD14++CD16− (M1), “intermediate” CD14++CD16+ (M2), and “non-classical” CD14+CD16++ (M3) monocytes reflects the activation state of these cells. The full spectrum of monocyte and its function is still unknown. The present pilot study describes the monocyte subpopulations and their human leukocyte antigen DR expression during the post-transplant period as well as during transplant-related adverse events of 30 pediatric patients and young adults with hemato-oncological malignancies and immunodeficiency disorders in comparison to healthy children and young adults. A significant change of the human leukocyte antigen DR expression in all three monocyte subpopulations during the period after bone marrow transplantation depending on the time after transplantation and adverse events could be recognized. Prior to and during sepsis or bacterial infection, a significant decrease in human leukocyte antigen DR expression occurred. A significant increase on CD14++CD16− monocytes could be observed during graft-versus-host disease. The alterations of human leukocyte antigen DR expression on the monocyte subpopulations during adverse events after hematopoietic stem cell transplantation may be a sign of changes in the capacity of these subpopulations. Moreover, human leukocyte antigen DR expression in monocyte subpopulations may be used to monitor treatment responses in these entities.






Similar content being viewed by others
References
Flieger D, Ziegler-Heitbrock HW (1988) Abnormal blood monocytes in chronic lymphocytic leukemia. Cancer Res 48(17):4812–4816
Petersen J, Drivsholm A, Brandt M, Ambjornsen A, Dickmeiss E (1989) B lymphocyte function in multiple myeloma: analysis of T cell- and monocyte-dependent antibody production. Eur J Haematol 42(2):193–201
Rawstron AC, Davies FE, Owen RG, English A, Pratt G, Child JA, Jack AS, Morgan GJ (1998) B-lymphocyte suppression in multiple myeloma is a reversible phenomenon specific to normal B-cell progenitors and plasma cell precursors. Br J Haematol 100(1):176–183
Koenig M, Huenecke S, Salzmann-Manrique E, Esser R, Quaritsch R, Steinhilber D, Radeke HH, Martin H, Bader P, Klingebiel T, Schwabe D, Schneider G, Lehrnbecher T, Orth A, Koehl U (2010) Multivariate analyses of immune reconstitution in children after allo-SCT: risk-estimation based on age-matched leukocyte sub-populations. Bone Marrow Transplant 45(4):613–621. doi:10.1038/bmt.2009.204
Barbalat R, Lau L, Locksley RM, Barton GM (2009) Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat Immunol 10(11):1200–1207. doi:10.1038/ni.1792
Serbina NV, Cherny M, Shi C, Bleau SA, Collins NH, Young JW, Pamer EG (2009) Distinct responses of human monocyte subsets to Aspergillus fumigatus conidia. J Immunol (Baltim Md 1950) 183(4):2678–2687. doi:10.4049/jimmunol.0803398
Serbina NV, Jia T, Hohl TM, Pamer EG (2008) Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol 26:421–452. doi:10.1146/annurev.immunol.26.021607.090326
Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, Scherberich J, Schmitz J, Shortman K, Sozzani S, Strobl H, Zembala M, Austyn JM, Lutz MB (2010) Nomenclature of monocytes and dendritic cells in blood. Blood 116(16):e74–e80. doi:10.1182/blood-2010-02-258558
Passlick B, Flieger D, Ziegler-Heitbrock HW (1989) Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 74(7):2527–2534
Gupta M, Mahanty S, Ahmed R, Rollin PE (2001) Monocyte-derived human macrophages and peripheral blood mononuclear cells infected with ebola virus secrete MIP-1alpha and TNF-alpha and inhibit poly-IC-induced IFN-alpha in vitro. Virology 284(1):20–25. doi:10.1006/viro.2001.0836
Stroher U, West E, Bugany H, Klenk HD, Schnittler HJ, Feldmann H (2001) Infection and activation of monocytes by Marburg and Ebola viruses. J Virol 75(22):11025–11033. doi:10.1128/jvi. 75.22.11025-11033.2001
Fingerle G, Pforte A, Passlick B, Blumenstein M, Strobel M, Ziegler-Heitbrock HW (1993) The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood 82(10):3170–3176
Blumenstein M, Boekstegers P, Fraunberger P, Andreesen R, Ziegler-Heitbrock HW, Fingerle-Rowson G (1997) Cytokine production precedes the expansion of CD14 + CD16+ monocytes in human sepsis: a case report of a patient with self-induced septicemia. Shock (Augusta Ga) 8(1):73–75
Allen JB, Wong HL, Guyre PM, Simon GL, Wahl SM (1991) Association of circulating receptor Fc gamma RIII-positive monocytes in AIDS patients with elevated levels of transforming growth factor-beta. J Clin Invest 87(5):1773–1779. doi:10.1172/jci115196
Locher C, Vanham G, Kestens L, Kruger M, Ceuppens JL, Vingerhoets J, Gigase P (1994) Expression patterns of Fc gamma receptors, HLA-DR and selected adhesion molecules on monocytes from normal and HIV-infected individuals. Clin Exp Immunol 98(1):115–122
Ancuta P, Kunstman KJ, Autissier P, Zaman T, Stone D, Wolinsky SM, Gabuzda D (2006) CD16+ monocytes exposed to HIV promote highly efficient viral replication upon differentiation into macrophages and interaction with T cells. Virology 344(2):267–276. doi:10.1016/j.virol.2005.10.027
Vanham G, Edmonds K, Qing L, Hom D, Toossi Z, Jones B, Daley CL, Huebner B, Kestens L, Gigase P, Ellner JJ (1996) Generalized immune activation in pulmonary tuberculosis: co-activation with HIV infection. Clin Exp Immunol 103(1):30–34
Ziegler-Heitbrock L (2007) The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol 81(3):584–592. doi:10.1189/jlb.0806510
Turner JD, Bourke CD, Meurs L, Mbow M, Dieye TN, Mboup S, Polman K, Mountford AP (2014) Circulating CD14brightCD16+ ‘intermediate’ monocytes exhibit enhanced parasite pattern recognition in human helminth infection. PLoS Negl Trop Dis 8(4):e2817. doi:10.1371/journal.pntd.0002817
Ziegler-Heitbrock HW (1996) Heterogeneity of human blood monocytes: the CD14+ CD16+ subpopulation. Immunol Today 17(9):424–428
Dayyani F, Belge KU, Frankenberger M, Mack M, Berki T, Ziegler-Heitbrock L (2003) Mechanism of glucocorticoid-induced depletion of human CD14 + CD16+ monocytes. J Leukoc Biol 74(1):33–39
Fingerle-Rowson G, Angstwurm M, Andreesen R, Ziegler-Heitbrock HW (1998) Selective depletion of CD14+ CD16+ monocytes by glucocorticoid therapy. Clin Exp Immunol 112(3):501–506
Xu PB, Lou JS, Ren Y, Miao CH, Deng XM (2012) Gene expression profiling reveals the defining features of monocytes from septic patients with compensatory anti-inflammatory response syndrome. J Infect 65(5):380–391. doi:10.1016/j.jinf.2012.08.001
Docke WD, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, Volk HD, Kox W (1997) Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med 3(6):678–681
Monneret G, Finck ME, Venet F, Debard AL, Bohe J, Bienvenu J, Lepape A (2004) The anti-inflammatory response dominates after septic shock: association of low monocyte HLA-DR expression and high interleukin-10 concentration. Immunol Lett 95(2):193–198. doi:10.1016/j.imlet.2004.07.009
Docke WD, Hoflich C, Davis KA, Rottgers K, Meisel C, Kiefer P, Weber SU, Hedwig-Geissing M, Kreuzfelder E, Tschentscher P, Nebe T, Engel A, Monneret G, Spittler A, Schmolke K, Reinke P, Volk HD, Kunz D (2005) Monitoring temporary immunodepression by flow cytometric measurement of monocytic HLA-DR expression: a multicenter standardized study. Clin Chem 51(12):2341–2347. doi:10.1373/clinchem.2005.052639
Döring M, Rohrer KM, Erbacher A, Gieseke F, Schwarze CP, Bader P, Handgretinger R, Hofbeck M, Kerst G (2014) Human leukocyte antigen DR surface expression on CD14+ monocytes during adverse events after hematopoietic stem cell transplantation. Ann Hematol. doi:10.1007/s00277-014-2185-y
Dayyani F, Joeinig A, Ziegler-Heitbrock L, Schmidmaier R, Straka C, Emmerich B, Meinhardt G (2004) Autologous stem-cell transplantation restores the functional properties of CD14 + CD16+ monocytes in patients with myeloma and lymphoma. J Leukoc Biol 75(2):207–213. doi:10.1189/jlb.0803386
Goldstein B, Giroir B, Randolph A (2005) International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc 6(1):2–8. doi:10.1097/01.pcc.0000149131.72248.e6
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, Lerner KG, Thomas ED (1974) Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 18(4):295–304
Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, Vogelsang GB, Sensenbrenner LL, Santos GW, Saral R (1987) Venoocclusive disease of the liver following bone marrow transplantation. Transplantation 44(6):778–783
McDonald GB, Sharma P, Matthews DE, Shulman HM, Thomas ED (1984) Venocclusive disease of the liver after bone marrow transplantation: diagnosis, incidence, and predisposing factors. Hepatology (Baltimore Md) 4(1):116–122
McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M, Hardin BJ, Shulman HM, Clift RA (1993) Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med 118(4):255–267
Vereyken EJ, Kraaij MD, Baan CC, Rezaee F, Weimar W, Wood KJ, Leenen PJ, Rowshani AT (2013) A shift towards pro-inflammatory CD16+ monocyte subsets with preserved cytokine production potential after kidney transplantation. PLoS One 8(7):e70152. doi:10.1371/journal.pone.0070152
Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, Jais JP, D'Cruz D, Casanova JL, Trouillet C, Geissmann F (2010) Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 33(3):375–386. doi:10.1016/j.immuni.2010.08.012
Strauss-Ayali D, Conrad SM, Mosser DM (2007) Monocyte subpopulations and their differentiation patterns during infection. J Leukoc Biol 82(2):244–252. doi:10.1189/jlb.0307191
Wittenbecher F, Rieger K, Dziubianau M, Herholz A, Mensen A, Blau IW, Uharek L, Dorken B, Thiel A, Na IK (2013) Rabbit antithymocyte globulin induces rapid expansion of effector memory CD8 T cells without accelerating acute graft versus host disease. Leuk Res Rep 2(2):82–85. doi:10.1016/j.lrr.2013.09.001
Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, Volin L, Ruutu T, Heim DA, Schwerdtfeger R, Kolbe K, Mayer J, Maertens JA, Linkesch W, Holler E, Koza V, Bornhauser M, Einsele H, Kolb HJ, Bertz H, Egger M, Grishina O, Socie G (2009) Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol 10(9):855–864. doi:10.1016/s1470-2045(09)70225-6
Socie G, Schmoor C, Bethge WA, Ottinger HD, Stelljes M, Zander AR, Volin L, Ruutu T, Heim DA, Schwerdtfeger R, Kolbe K, Mayer J, Maertens JA, Linkesch W, Holler E, Koza V, Bornhauser M, Einsele H, Kolb HJ, Bertz H, Egger M, Grishina O, Finke J (2011) Chronic graft-versus-host disease: long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti-T-cell globulin ATG-Fresenius. Blood 117(23):6375–6382. doi:10.1182/blood-2011-01-329821
Reinhardt K, Foell D, Vogl T, Mezger M, Wittkowski H, Fend F, Federmann B, Gille C, Feuchtinger T, Lang P, Handgretinger R, Andreas Bethge W, Holzer U (2014) Monocyte-induced development of Th17 cells and the release of S100 proteins are involved in the pathogenesis of graft-versus-host disease. J Immunol (Baltim Md 1950) 193(7):3355–3365. doi:10.4049/jimmunol.1400983
Betts BC, St Angelo ET, Kennedy M, Young JW (2011) Anti-IL6-receptor-alpha (tocilizumab) does not inhibit human monocyte-derived dendritic cell maturation or alloreactive T-cell responses. Blood 118(19):5340–5343. doi:10.1182/blood-2011-06-363390
DiCarlo J, Agarwal-Hashmi R, Shah A, Kim P, Craveiro L, Killen R, Rosenberg-Hasson Y, Maecker H (2014) Cytokine and chemokine patterns across 100 days after hematopoietic stem cell transplantation in children. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant 20(3):361–369. doi:10.1016/j.bbmt.2013.11.026
Takeyama N, Yabuki T, Kumagai T, Takagi S, Takamoto S, Noguchi H (2007) Selective expansion of the CD14(+)/CD16(bright) subpopulation of circulating monocytes in patients with hemophagocytic syndrome. Ann Hematol 86(11):787–792. doi:10.1007/s00277-007-0332-4
Sester U, Sester M, Heine G, Kaul H, Girndt M, Kohler H (2001) Strong depletion of CD14(+)CD16(+) monocytes during haemodialysis treatment. Nephrol Dial Transplant Off Publ Eur Dial Transplant Assoc Eur Renal Assoc 16(7):1402–1408
Heron M, Grutters JC, van Velzen-Blad H, Veltkamp M, Claessen AM, van den Bosch JM (2008) Increased expression of CD16, CD69, and very late antigen-1 on blood monocytes in active sarcoidosis. Chest 134(5):1001–1008. doi:10.1378/chest. 08-0443
Schlitt A, Heine GH, Blankenberg S, Espinola-Klein C, Dopheide JF, Bickel C, Lackner KJ, Iz M, Meyer J, Darius H, Rupprecht HJ (2004) CD14 + CD16+ monocytes in coronary artery disease and their relationship to serum TNF-alpha levels. Thromb Haemost 92(2):419–424. doi:10.1267/thro04080419
Kawanaka N, Yamamura M, Aita T, Morita Y, Okamoto A, Kawashima M, Iwahashi M, Ueno A, Ohmoto Y, Makino H (2002) CD14+, CD16+ blood monocytes and joint inflammation in rheumatoid arthritis. Arthritis Rheum 46(10):2578–2586. doi:10.1002/art.10545
Berg KE, Ljungcrantz I, Andersson L, Bryngelsson C, Hedblad B, Fredrikson GN, Nilsson J, Bjorkbacka H (2012) Elevated CD14++CD16- monocytes predict cardiovascular events. Circ Cardiovasc Genet 5(1):122–131. doi:10.1161/circgenetics.111.960385
Rogacev KS, Cremers B, Zawada AM, Seiler S, Binder N, Ege P, Grosse-Dunker G, Heisel I, Hornof F, Jeken J, Rebling NM, Ulrich C, Scheller B, Bohm M, Fliser D, Heine GH (2012) CD14++CD16+ monocytes independently predict cardiovascular events: a cohort study of 951 patients referred for elective coronary angiography. J Am Coll Cardiol 60(16):1512–1520. doi:10.1016/j.jacc.2012.07.019
Kim OY, Monsel A, Bertrand M, Coriat P, Cavaillon JM, Adib-Conquy M (2010) Differential down-regulation of HLA-DR on monocyte subpopulations during systemic inflammation. Crit Care (Lond Engl) 14(2):R61. doi:10.1186/cc8959
Peter J, Frey O, Stallmach A, Bruns T (2013) Attenuated antigen-specific T cell responses in cirrhosis are accompanied by elevated serum interleukin-10 levels and down-regulation of HLA-DR on monocytes. BMC Gastroenterol 13:37. doi:10.1186/1471-230x-13-37
Azeredo EL, Neves-Souza PC, Alvarenga AR, Reis SR, Torrentes-Carvalho A, Zagne SM, Nogueira RM, Oliveira-Pinto LM, Kubelka CF (2010) Differential regulation of toll-like receptor-2, toll-like receptor-4, CD16 and human leucocyte antigen-DR on peripheral blood monocytes during mild and severe dengue fever. Immunology 130(2):202–216. doi:10.1111/j.1365-2567.2009.03224.x
Conflict of interest
All authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Döring, M., Cabanillas Stanchi, K.M., Haufe, S. et al. Patterns of monocyte subpopulations and their surface expression of HLA-DR during adverse events after hematopoietic stem cell transplantation. Ann Hematol 94, 825–836 (2015). https://doi.org/10.1007/s00277-014-2287-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-014-2287-6