Abstract
Background and Purpose
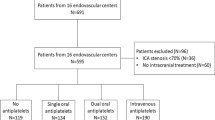
Data on safety and efficacy of periprocedural use of heparin are limited during treatment of acute ischemic stroke patients with anterior circulation tandem occlusion. This study aimed to investigate the impact of heparin use during endovascular therapy of anterior circulation tandem occlusions on the functional and safety outcomes.
Methods
A retrospective analysis of the multicenter observational TITAN registry was performed. Patients with anterior circulation tandem occlusion and treated with endovascular therapy (EVT) were included, with or without extracranial carotid intervention. We divided patients into two groups based on periprocedural heparin use (heparin vs. non-heparin). The dose of intravenous unfractionated heparin ranged from 1500 to 2500 I.U. Primary study endpoint was 90-day Modified Rankin Scale (mRS). Secondary study endpoint included angiographic and safety endpoints such as hemorrhagic complications. A propensity-score-matched analysis was performed.
Results
Among 369 patients, heparin was used in 68 patients (18.4%). In the propensity-score-matched cohort, favorable outcome (mRS 0–2) occurred in 51.3% in heparin group and 58.0% in non-heparin group (matched OR, 0.76; 95% CI, 0.32–1.78; P = 0.52). Similar result was found in propensity-score-adjusted cohort (adjusted OR, 0.72; 95%CI, 0.39–1.32; P = 0.28). Likewise, there was no difference in the rate of successful reperfusion (mTICI 2b-3) (propensity-score-adjusted OR, 1.03; 95%CI, 0.50–2.09; P = 0.93) neither in safety endpoints between the two groups.
Conclusions
Periprocedural heparin use during EVT of anterior circulation tandem occlusions was not associated with better functional, angiographic or safety outcomes. These findings are applicable for low doses of heparin, and further studies are warranted.



Similar content being viewed by others
References
Bracard S, Ducrocq X, Mas JL, Soudant M, Oppenheim C, Moulin T, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15:1138–47.
van de Graaf RA, Chalos V, Del Zoppo GJ, van der Lugt A, Dippel DWJ, Roozenbeek B. Periprocedural antithrombotic treatment during acute mechanical thrombectomy for ischemic stroke: a systematic review. Front Neurol. 2018;9:238.
Winningham MJ, Haussen DC, Nogueira RG, Liebeskind DS, Smith WS, Lutsep HL, et al. Periprocedural heparin use in acute ischemic stroke endovascular therapy: the TREVO 2 trial. J Neurointerv Surg. 2018;10:611–4.
Nahab F, Walker GA, Dion JE, Smith WS. Safety of periprocedural heparin in acute ischemic stroke endovascular therapy: the multi MERCI trial. J Stroke Cerebrovasc Dis. 2012;21:790–3.
Gory B, Haussen DC, Piotin M, Steglich-Arnholm H, Holtmannspötter M, Labreuche J, et al. Impact of intravenous thrombolysis and emergent carotid stenting on reperfusion and clinical outcomes in patients with acute stroke with tandem lesion treated with thrombectomy: a collaborative pooled analysis. Eur J Neurol. 2018;25:1115–20.
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46:399–424.
Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med. 2014;33:1057–69.
Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–107.
Mattei A. Estimating and using propensity score in presence of missing background data: an application to assess the impact of childbearing on wellbeing. Stat Methods Appl. 2008;18:257–73.
van Buuren. Multivariate imputation by chained equations in R. J Stat Softw. 2011;45. Available from: https://www.jstatsoft.org/article/view/v045i03.
Rubin DB. Multiple imputation for nonresponse in surveys. New York: Wiley; 2004 (Ch. 3).
Zhu F, Labreuche J, Haussen DC, Piotin M, Steglich-Arnholm H, Holtmannspötter M, et al. Hemorrhagic transformation after thrombectomy for tandem large vessel occlusion strokes: incidence, predictors, and clinical implications. Stroke. 2019;50:516–9.
Nahab F, Kass-Hout T, Shaltoni HM. Periprocedural antithrombotic strategies in acute ischemic stroke interventional therapy. Neurology. 2012;79:174–81.
Funding
This work was supported by Stryker.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
Dr. Piotin reports grants from penumbra, personal fees from Medtronic, other from Stryker, other from Microvention, other from Balt, outside the submitted work. Dr. Taschner reports grants and non-financial support from Microvention, personal fees and non-financial support from Stryker, non-financial support from Acandis, personal fees and non-financial support from Neuravi, non-financial support from Medtronic, outside the submitted work. Dr. Nogueira reports non-financial support from Stryker, non-financial support from Medtronic, non-financial support from Penumbra, outside the submitted work. Dr. Papanagiotou reports grants from Medtronic, non-financial support from Penumbra Inc., non-financial support from Johnson & Johnson, outside the submitted work. Dr. Siddiqui reports grants from National Institutes of Health/NINDS/NIBIB, University at Buffalo, personal fees from Hotspur, Intratech Medical, StimSox, Valor Medical, Blockade Medical and Lazarus Effect;, non-financial support from Codman & Shurtleff, Inc, Concentric Medical, ev3/Covidien Vascular Therapies, GuidePoint Global Consulting, Penumbra, Stryker, Pulsar Vascular, Microvention, Lazarus Effect, Blockade Medical, other from null, outside the submitted work. Dr. Lapergue reports non-financial support from Medtronic, non-financial support from Penumbra, outside the submitted work. Dr. Cognard reports personal fees from Medtronic, personal fees from Microvention, personal fees from Stryker, outside the submitted work. Dr. Spiotta reports non-financial support from Penumbra, non-financial support from Pulsa Vascular, non-financial support from Microvention, non-financial support from Stryker, outside the submitted work. Dr. Mazighi reports non-financial support from Medtronic, non-financial support from Boehringer Ingelheim, non-financial support from Bayer, non-financial support from Servier, outside the submitted work. Dr. Biondi reports non-financial support from Balt, non-financial support from Medtronic, non-financial support from Microvention, non-financial support from Stryker, non-financial support from Phenox, outside the submitted work. Dr. Turjman reports grants from Stryker, during the conduct of the study, non-financial support from Stryker, non-financial support from Medtronic, non-financial support from Johnson & Johnson, non-financial support from Balt, outside the submitted work. Dr. Gory reports grants from Stryker, personal fees from Medtronic, non-financial support from Balt, during the conduct of the study. François Zhu, Dr Steglich-Arnholm, Julien Labreuche, Dr Holtmannspötter, Dr Eiden,Dr Haussen, Dr Boutchakova, Dr Dorn, Dr Killer, Dr Mangiafico, Dr Ribo, Dr Psychogios, Dr Anadani, Dr Labeyrie, Dr Richard, Dr Anxionnat, Dr Bracard have nothing to disclose.
Informed Consent
The local institutional review boards approved the study. Informed consent was not required as this was a retrospective analysis. All data and materials have been made publicly available in a public repository and can be accessed by a request from Dr. Benjamin Gory, MD, Ph.D.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of TITAN (Thrombectomy In TANdem Lesions) investigators are given in the Appendix section.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Appendix: TITAN (Thrombectomy in TANdem Lesions) Investigators
Appendix: TITAN (Thrombectomy in TANdem Lesions) Investigators
Andreas Kastrup, Jonathan Andrew Grossberg, Adrien Guenego, Julien Darcourt, Isabelle Vukasinovic, Elisa Pomero, Jason Davies, Leonardo Renieri, Corentin Hecker, Maria Muchada, Arturo Consoli, Georges Rodesch, Emmanuel Houdart, Raymond Turner, Aquilla Turk, Imran Chaudry, Johanna Lockau, Raphaël Blanc, Hocine Redjem, Daniel Behme, Hussain Shallwani, Maurer Christopher, Anne-Laure Derelle, Romain Tonnelet, Liang Liao, Lisa Humbertjean, Gioia Mione, Jean-Christophe Lacour.
Rights and permissions
About this article
Cite this article
Zhu, F., Piotin, M., Steglich-Arnholm, H. et al. Periprocedural Heparin During Endovascular Treatment of Tandem Lesions in Patients with Acute Ischemic Stroke: A Propensity Score Analysis from TITAN Registry. Cardiovasc Intervent Radiol 42, 1160–1167 (2019). https://doi.org/10.1007/s00270-019-02251-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-019-02251-4