Abstract
Background
While the metabolic syndrome (MS) is being recognized as an important risk factor for intrahepatic cholangiocarcinoma (ICC), the outcomes of liver resection in this context remain poorly described. This study aims to report the short- and long-term results of hepatectomy for patients with MS as risk factor for the development of ICC (MS+).
Methods
All patients undergoing hepatectomy for ICC between 2000 and 2016 at a single center were retrospectively analyzed. The perioperative outcomes of MS+ and ICC patients without MS (MS−) were compared.
Results
Among 115 resected ICC patients, 40 (34.8%) were MS+ and 75 (65.2%) were MS−. MS+ exhibited an increased Charlson comorbidity index (5 ± 2 vs. 2 ± 2, p < 0.001) than MS− patients. While operative characteristics did not differ significantly between the 2 groups, MS+ experienced higher rate of major complications (62.5 vs. 29.3%, p = 0.001). On multivariate analysis, MS+ was an independent risk factor of major complication (HR 2.86, 95% CI 1.07–7.60, p = 0.036) and major cardiorespiratory complication (HR 4.35, 95% CI 1.50–12.62, p = 0.007). Pathological analysis revealed that MS+ displayed higher rates of non-alcoholic fatty liver disease (60.0 vs. 31.1%, p = 0.003) and non-alcoholic steatohepatitis (25 vs. 5.4%, p = 0.005). MS+ was independently associated with decreased risk of recurrence (HR 0.47, 95% CI 0.26–0.85, p = 0.001).
Conclusions
MS+ accounts for 35% of resected ICC patients. The existence of significant cardiovascular comorbidities increases postoperative morbidity and requires specific management.
Similar content being viewed by others
Introduction
The metabolic syndrome (MS), which comprises a cluster of metabolic risk factors including increased fasting glucose, central obesity, dyslipidemia and hypertension, is an increasingly important health problem in the USA and in Western countries where its prevalence may be as high as 25% [1]. As a consequence, non-alcoholic fatty liver disease (NAFLD), which represents the hepatic manifestation of the MS, has turned into one of the most prominent liver diseases in Western countries [2]. Moreover, the MS itself but also its individual components have been shown to promote the development of various gastrointestinal malignancies [3] including hepatocellular carcinoma (HCC) [4] and more recently intrahepatic cholangiocarcinoma (ICC) [5] [6]. HCC in a context of MS does not necessarily develop on a background of severe fibrosis [7]. Indeed, the development of HCC in a setting of MS does not seem to follow the classical dysplasia–carcinoma sequence [8]. As a matter of fact, MS and NAFLD microenvironment activate oncogenic pathways and accelerate the development of HCC through parallel effects and complex interactions of local and systemic inflammation, insulin resistance and lipotoxicity before cirrhosis occurs [9]. Altogether, past decade literature has thus suggested that MS-related HCC displayed specific histological features, prognosis and required specific surgical management [10].
ICC is the second most common primary liver cancer but remains an uncommon and enigmatic tumor [11]. Although the incidence of ICC worldwide is considerably lower than that of HCC, several recent studies have reported troubling increasing incidence of ICC over the last few decades [12]. On the one hand, classical factors associated with chronic biliary inflammation, such as hepatobiliary flukes, primary sclerosing cholangitis, biliary tract cysts, hepatolithiasis and toxins, which are known to promote ICC [11], are only marginally observed. On the other hand, the stable incidence of other recognized risk factors for ICC, including cirrhosis, chronic hepatitis B and C infections, and alcohol [13] cannot account for the increasing incidence of this lesion. Finally, while it has been suggested that ICC was associated with NAFLD, obesity and diabetes [5, 6, 14, 15] this entity nevertheless remains poorly characterized and understood.
By analogy with HCC, it may be speculated that a significant number of ICC without classical risk factors could occur in a context of MS, which could explain the rising incidence of ICC in Western countries. Similarly, it could be anticipated that patients with MS-related ICC could exhibit specific operative risk and long-term outcomes [10] [16]. Hence, the present study aimed at comparing the characteristics, postoperative outcomes and long-term results after surgical resection of ICC occurring in a context of MS (MS+) with those of standard ICC (MS−).
Methods
Study population
All patients undergoing partial liver resection (LR) for ICC between 2000 and 2016 at Beaujon Hospital, Clichy, France, were included in the present study.
Most pre-, per- and postoperative data, pathological features and long-term outcomes were retrieved from a prospectively implemented institutional database and retrospectively reviewed. To ensure the most accurate data collection as possible, especially regarding metabolic syndrome risk factors and survival data, all patients alive at the time of the study were also contacted for this study. The main analysis focused on the comparison of short- and long-term results between MS+ and MS− patients. To highlight the specific impact of the MS on the postoperative course and the prognosis without interference of a potential abnormal underlying liver parenchyma, a subgroup analysis excluding patients with NASH and/or severe fibrosis was then performed.
Risk factors for ICC retained in this study included the MS, parasites, intrahepatic stones, primary sclerosing cholangitis, hepatitis B virus infection (HBV), intra-epithelial neoplasia (BiliN, PaniN), and history of underlying fibrotic liver disease regardless the etiology. The diagnosis of MS was considered when three or more of the following criteria were present [17]: central obesity; dyslipidemia (triglycerides 1.7 mmol/l or above, or high-density lipoprotein cholesterol less than 1.03 mmol/l in men or less than 1.29 mmol/l in women); type II diabetes or glucose intolerance with fasting glucose 5.6 mmol/l or above; and hypertension (blood pressure above 135/85 mmHg). Because of the retrospective nature of the study, it was assumed that central obesity was reached when the patient’s body mass index was greater than 28 kg/m2, as described previously [18]. Similarly, it was assumed that patients receiving statin or fenofibrate medication had dyslipidemia. Chronic alcohol consumption was defined according to the WHO definition as a consumption > 30 g/day in men and > 20 g/day in women. Patients with incomplete data about MS and outcomes (n = 5) were not included in this study.
Preoperative evaluation
Preoperative investigations included complete blood and liver function tests as well as routine cardiorespiratory evaluation. Computed tomography (CT) and magnetic resonance imaging (MRI) were performed to assess both underlying liver and tumor characteristics. Preoperative percutaneous biopsy of the non-tumorous parenchyma was performed when an abnormal underlying liver parenchyma was suspected. In patients requiring a resection with an anticipated future liver remnant liver <30% of the total liver volume in case of normal underlying liver and <40% in the presence of severe fibrosis, portal vein embolization (PVE) was performed followed with evaluation of the liver hypertrophy by follow-up CT scan 3–4 weeks later [19].
Surgical procedure
All resections were performed with curative intent by four seniors hepatobiliary surgeons (OS, JB, SD, OF). Major liver resection was defined as resection of three or more Couinaud’s segments [20]. The laparoscopic approach was performed on a case-by-case basis since 2008. For all procedures, liver transection was performed with the crush/clamp technique or ultrasonic dissection under low central venous pressure (less than 5 mmHg) as described previously [21]. Intermittent pedicle clamping was performed in case of bleeding, or routinely in some patients in order to obtain a bloodless operative field. Lymphadenectomy of the hepatoduodenal ligament was performed selectively according to preoperative CT scan and peroperative findings.
Postoperative outcomes
Following hepatic resection, patients were seen daily by a physician until hospital discharge. A contrast enhanced thoraco-abdominopelvic CT scan was performed on postoperative day (POD) 7 or earlier in suspected cases of abdominal or pulmonary complication. Specific liver complications, encountered more often after major liver procedures, were detailed as follows: liver failure was defined according to the “50–50 criteria” on POD 5 [22]; ascites was defined as an abdominal drainage output of more than 10 ml/kg/day after the third POD [23]; and bile leakage was defined by a bilirubin concentration in the drainage fluid more than threefold higher than that in serum [24]. Respiratory complications were defined as the development of one or more of the followings: pulmonary infection, symptomatic pleural effusion, respiratory insufficiency, acute respiratory distress syndrome and pulmonary embolism. Pulmonary infection was defined by an alteration of chest radiography with/without positive sputum cultures with/without CT scan results associated with fever and hyperleukocytosis [25]. Pleural effusion was diagnosed by chest radiography with/without CT scan. Symptomatic pleural effusion was defined as pleural effusion requiring oxygen therapy management. Patients were defined as having respiratory insufficiency if the total duration of ventilator-assisted respiration during the postoperative hospital stay exceeded 48 h. Pulmonary embolism was confirmed by thoracic CT scan. Cardiac complications were defined as the development of one or more of the followings: acute coronary syndrome, rhythm disorder, conduction disorder, cardiac failure, aortic dissection, hypertensive crisis, pericarditis, pericardial tamponade. Postoperative complications were stratified according to the Dindo–Clavien classification [26], which defines major complications by a grade of IIIa or more. Major cardiorespiratory or liver-related complications were defined as any cardiorespiratory and liver-related complication with a Dindo–Clavien grade of IIIa or more. The comprehensive complication index (CCI) [27] was assessed for each patient using a dedicated automated online calculator (http://www.assessurgery.com/calculator_single/). Complications and operative mortality were considered as those occurring within 90 day after surgery, or at any time during the postoperative hospital stay.
Pathological analysis
For the purpose of this study, all resected liver specimens were specifically reviewed by a single pathologist specialized in liver diseases (NP). Main tumor characteristics, including size, macroscopic type according to the World Health Organization classification of ICC [28] (mass forming, periductal infiltrating, periductal infiltrating and mass forming, intraductal growth), number of nodules, satellite nodules (considered as lesions occurring within 2 cm of the main tumor), vascular invasion, perineural invasion and grade of differentiation, were assessed. Tumor T status, N status and M status were established from the pathological analysis and classified according to the eighth edition of the AJCC staging system [29]. The AJCC eight edition stage was assigned only for the patients with available N status. Liver parenchyma was assessed for the presence of fibrosis, staged from 0 to 4 according to the NASH Clinical Research Network Scoring System [30], and considered severe in patients with stages F3 and F4. NAFLD was defined by the presence of steatosis in >5% of hepatocytes, and its histological features were collected [30]. NAFLD lesions were graded according to the SAF (steatosis, activity, fibrosis) score [31], including macrovesicular steatosis (on a scale of 0–3), lobular inflammation and hepatocellular ballooning (each on a scale of 0–2). The FLIP algorithm was used to define the presence of NASH [32]. Liver parenchyma was considered abnormal in patients with severe underlying fibrosis (stage F3 or F4) or in those without severe underlying fibrosis but with NAFLD.
Statistical analysis
Quantitative variables are expressed as mean (±standard deviation) or as median (interquartile 25–75). Qualitative variables are expressed as percentages. The Mann–Whitney U test or Kruskal–Wallis test were used for comparisons of quantitative variables as appropriate, whereas a Chi-square test or Fisher exact test was used to compare categorical data. The probability of developing major complications was estimated using a multivariate logistic regression model. All variables that differed significantly (p < 0.1) when comparing the 2 groups were included in the logistic model, and backward selection was applied. The Kaplan–Meier method was used to estimate survival probabilities, which were compared using the Log-rank test. The date of the patient’s last contact was used as the end of follow-up in all censored patients. No patient was lost to follow-up. Follow-up was updated until February 2017. Postoperative deaths were included in the overall survival (OS) analysis but excluded from the recurrence-free survival (RFS) analysis. Multivariate analysis was performed using a Cox proportional hazard model to identify independent prognostic factors for OS and RFS. A p value <0.05 was considered significant for all tests. The variable “Lymph node metastasis” was integrated in the Cox model according to 3 modalities after the pathological analysis: absence of lymph node metastasis or N0, presence of lymph node metastasis or N+, no available nodal status or Nx. Subgroup analysis was performed to rule out the effect of NASH and underlying fibrosis between MS+ and MS− patients. Hence, MS+ patients without significant fibrosis or NASH (sMS+) were compared to MS− patients without NASH or significant fibrosis (sMS−). All statistical analyses were performed with SPSS version 20.0 (IBM Corp., Armonk, NY).
Results
Patients’ characteristics and surgical procedures
A total of 115 patients were resected for ICC during the study period including 40 (34.8%) MS+ patients and 75 (65.2%) MS− patients. Among these latter, 33 (28.7%), 27 (23.5%) and 17 (14.8%) patients had none, one and two criteria for the MS, respectively. The comparison of the baseline characteristics of the 2 groups is provided in Table 1. Briefly, several characteristics bound to the MS such as a history of cardiovascular disease (37.5% vs. 16.0%, p = 0.010), use of antiplatelet agents (27.5% vs. 5.3%, p = 0.001), oral antidiabetic (42.5% vs. 2.7%, p = 0.001) and lipid-lowering drugs (65% vs. 9.3%, p < 0.001) were more frequently observed in MS+ patients. MS+ patients had increased anesthetic risk as shown by the higher rate of patients with an ASA score of 3 or greater (37.5% vs. 5.3%, p < 0.001) and increased Charlson comorbidity index (5 ± 2 vs. 2 ± 2, p < 0.001) [33]. Operative characteristics were not significantly different between the 2 groups except for the rate of intra-operative transfusion, which was higher in MS+ patients (32.5% vs. 13.3%, p = 0.014).
Postoperative outcomes and risk factors for major postoperative complications
The details of the postoperative course of MS+ and MS− patients are provided in Table 2. The overall postoperative mortality rate was 11.3%. Even though MS+ patients had a higher postoperative mortality rate than MS− patients (17.5% vs. 8.0%, p = 0.136) the difference did not reach significance. In the MS+ group, the causes of death were due to liver-related complications in 2 patients and due to non-liver-related complications in 5 patients (1 cardiovascular collapse during dialysis, 1 acute myocardial infarction and 3 acute respiratory distress syndromes related to pulmonary infections). In the MS− group, the causes of death were due to liver-related complications in 2 patients and to non-liver-related complications in 4 patients (1 pleural empyema and 1 acute respiratory distress syndrome over pulmonary infection, 1 peritonitis and 1 lower limb compartment syndrome).
Compared with MS− patients, MS+ patients experienced significantly increased rates of overall major (29.3% vs. 62.5%, p = 0.001), and cardiovascular complications (17.3% vs. 52.5%, p < 0.001), had higher mean CCI (26 ± 28 vs. 42 ± 34, p = 0.007), but did not experience significantly different rates of major liver-related complications (18.7% vs. 27.5%, p = 0.274).
Univariate and multivariate analyses of the factors associated with major overall complications and major cardiorespiratory complications are displayed in Table 3 and Supplementary Table 1, respectively. The MS was an independent risk factor for both overall major complications (HR 2.86, 95% CI 1.07–7.560, p = 0.036) and major cardiorespiratory complications (HR 4.36, 95% CI 1.50–12.62, p = 0.007).
Pathological analysis
Tumor characteristics and details regarding the non-tumorous underlying liver are shown in Table 4. While MS+ patients had smaller tumors than MS− patients (64 ± 42 mm vs. 76 ± 36 mm, p = 0.048), other tumor characteristics were comparable between the 2 groups. According the AJCC eight edition, MS+ and MS− patients shared similar T stages and N status. Among the patients who had lymphadenectomy, MS+ and MS− disease’s staging was comparable. On the opposite, several significant differences including the rate of NAFLD and NASH were observed regarding the characteristics of the underlying non-tumorous liver.
Survival analysis, long-term outcomes and prognostic factors
Median follow-up was 21 months (IQR 10–42 months) and the 1-year, 3-year and 5-year OS of the whole series were 77.6%, 55.1% and 36.3%, respectively (median survival 42 (23–61) months). At last follow-up, 56 (48.7%) patients were deceased and 69 (60.0%) had experienced recurrence, involving the liver in 41 (59.4%) patients and extra-hepatic structures in 34 (50.0%) patients. The 1-year, 3-year and 5-year RFS of the whole series were 50.5%, 26.5% and 17.0%, respectively (median RFS 13 (7–19) months).
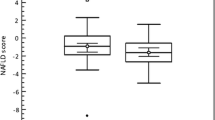
Median follow-up was comparable between the 2 groups (20 vs. 21 months, p = 0.596). While MS+ and MS− patients displayed non-significantly different overall survivals (median OS of 31 (0–79) vs. 42 (25–59) months; p = 0.830), MS+ patients had significantly better RFS than MS− patients (median RFS of 35 (0–76) vs. 12 (8–17) months; p = 0.011, Fig. 1).
Details of multivariate analyses for survival are reported in Tables 5 and 6. On multivariate analysis, the MS was independently associated with decreased risk of recurrence (HR 0.47, 95% CI 0.26–0.85, p = 0.013), while major resection (HR 3.03, 95% CI 1.39–6.48, p = 0.005), tumor size > 50 mm (HR 2.04, 95% CI 1.08–3.86), perineural invasion (HR 1.75, 95% CI 1.07–2.85, p = 0.026) and satellites nodules (HR 2.01, 95% CI 1.19–3.40, p = 0.009) were independently associated with increased risk of recurrence. On the opposite, the MS was not independently associated with OS.
Subgroup analysis after exclusion of the patients with NASH or severe fibrosis
Subgroup analysis was performed to rule out the effect of NASH and underlying fibrosis between MS+ and MS− patients. Hence, MS+ patients without significant fibrosis or NASH (sMS+) were compared to MS− patients without NASH or significant fibrosis (sMS−).
After exclusion of patients with NASH or severe (F3–F4) fibrosis, 23 patients (57.5% of the MS+ group) constituted the sMS+ group and 59 patients (78.7% of the MS− group) constituted the sMS− group. Details of the patients’ characteristics and operative characteristics are displayed in Supplementary Table 2. Similarly to the main analysis, sMS+ patients differed with the sMS− regarding MS components and characteristics bound to the MS.
In the subgroup analysis, the postoperative 90-day mortality rate was 6.1% and was not significantly different between the 2 subgroups (8.7% vs. 5.1%, p = 0.616). The comparison of the postoperative outcomes in the subgroup analysis population is detailed in Supplementary Table 3. sMS+ patients experienced an increased rate of major cardiorespiratory complications (43.5% vs. 16.9%, p = 0.012) associated with a trend toward an increased rate of major complications (52.2 vs. 30.5%, p = 0.067) but a similar CCI (34, IQR (8–52) vs. 21 IQR (0–38), p = 0.186) compared with sMS− patients.
The details of the pathological subgroup analysis are summarized in Supplementary Table 4. sMS− patients displayed higher rate of normal underlying liver (66.1% vs. 39.1%, p = 0.026) than sMS+ patients, while sMS+ patients more frequently displayed features of NAFLD (52.2% vs. 26.7%, p = 0.036) than sMS− patients.
The 1-year , 3-year and 5-year OS of the sMS+ and sMS− patients were 77.1%, 49.5% and 49.5%, and 81.1%, 56.7%, 23.9% (p = 0.455), respectively. sMS+ patients kept a significant advantage regarding RFS (55.6%, 42.3%, 33.9% vs. 42.8%, 12.8% and 2.6%, p = 0.002) (Supplementary Figure 1).
Discussion
In this study, which compared the perioperative characteristics of ICC patients with and without the MS, MS+ patients were at significantly higher risk of postoperative major complications, especially, cardiorespiratory complications but showed improved RFS survival compared with MS− patients.
In the present series, 35% of all resected ICC patients displayed a MS. By analogy to what has been reported for HCC [10, 16], this result supports the close relationship between the MS and ICC [6]. In this study, 60%, 25% and 30% of MS patients displayed NAFLD, NASH and severe fibrosis, respectively. These results suggest that the MS may first promote the development of ICC through underlying liver parenchymal damage related to the development of NAFLD. On the opposite, the fact that almost one quarter of MS+ patients exhibited strictly normal underlying liver parenchyma supports that ICC may also occur independently of any underlying liver parenchymal damage, as a consequence of insulin resistance-mediated systemic inflammation or following protumoral effects related to changes in composition of visceral fat, as recently suggested for HCC [34].
In the current study, MS+ patients experienced significantly higher rates of major complications and higher CCI than MS− patients. In addition, the MS tended to impact the mortality rate of the MS+ patients. Surprisingly, MS+ patients did not experience higher rates of major liver-related complications, especially liver failure despite higher incidence of abnormal underlying liver parenchyma changes. Of course, this result may be the consequence of a non-significant trend choosing parenchymal-sparing resection in MS+ patients. Yet, it could also be related to a wide use of preoperative underlying liver biopsy and subsequent PVE in patients requiring major right-sided resections with NASH even in the absence of severe fibrosis, who should be regarded at higher risk of postoperative liver decompensation [19]. Interestingly, the increase in major complications observed in MS+ patients mainly involved cardiorespiratory complications. It has been suggested that NAFLD patients were at higher risk of coronary artery calcifications, which could partly explain the higher rates of postoperative cardiac events in MS+ patients [35]. Yet, subgroup analysis suggested that even without NASH and fibrosis, MS+ patients still experienced higher postoperative cardiorespiratory complications than MS− patients. As a systemic disease, the MS itself is associated with a greater risk of perioperative complications after liver surgery [36] regardless the existence of NAFLD. In addition, MS patients frequently accumulate obesity, diabetes and hypertension. Obesity may negatively impact the postoperative course of patient undergoing liver resection [37]. Likewise diabetes has been shown to be associated with postoperative pulmonary complications [38]. Altogether the focus on MS patients in the present study clearly defines a population at high cardiorespiratory morbidity with shorter predicted survival, as reflected by the observed increased Charlson comorbidity index and the higher rate of patients with an ASA score of three or more. In this setting and similarly to what was suggested in MS-related HCC patients [10], MS+ ICC patients should therefore require specific management during the perioperative period.
The survival analysis revealed that despite similar OS, MS+ patients yielded better RFS survival than MS− patients. Furthermore, multivariate analysis showed that the presence of MS was the only protective factor for recurrence. On pathological examination, MS+ and MS− patients shared similar tumor characteristics apart from tumor size. Since MS+ ICC develop more frequently on a background of NAFLD, these patients are more likely to show abnormal liver tests [39]. Hence, the smaller size of the lesions in MS+ patients may account for an earlier diagnosis in MS+ patients, which could partly explain the observed improved better RFS [5] [40]. However, the survival analysis restrained to the early stage patients reported that MS+ patients kept a significant survival advantage than MS− patients regarding the RFS. Considering the high perioperative risk of MS+ patients but the observed favorable long-term outcomes, this study highlights that MS+ patients could benefit from the less aggressive surgical management, such as strategies of parenchymal sparing with margin clearance ≥ 5 mm [41] and/or laparoscopic approach if adequate lymphadenectomy can be performed [42]. In addition, given the reported favorable results of thermal ablation for unresectable ICC (1-year, 3-year and 5-year overall survival rates of 82%, 47% and 24%) [43], the current study raises the question of performing local destruction for small lesions in high risk MS+ patients. Another hypothesis, to explain the favorable prognosis of the MS+ patients, would be that, similarly to ICC occurring on chronic liver disease, MS-related ICCs more frequently exhibit some hypervascular pattern on CT scan than MS− ICC. These ICCs are known to have a more favorable oncologic prognosis [44] [45]. Nonetheless, the current study failed to fully elucidate why MS+ patients achieved more favorable long-term outcomes than MS− patients.
The present study has several limitations owing to both its retrospective nature and the small number of patients included over a long-time period. First, a higher rate of patients with the metabolic syndrome was observed during the more recent years of the study. This finding may be due to inaccurate data collection in MS parameters in the early part of the study period. Even though an attempt to contact all patients was undertaken, it is possible that some deceased patients at the time of contact may have been incorrectly categorized. Second, almost 50% of MS+ patients did not undergo pedicular lymphadenectomy [46]. While pedicular lymphadenectomy is currently recommended in patients with ICC resection, it is routinely performed in only 45% of cases [46]. The more favorable prognosis of hypervascular ICC is known to account for a decreased rate of lymph node metastases [47], and the lack of the N status could be a confusing factor regarding the analysis of the prognostic factors. The present study could not determine whether MS+ ICC did in fact belong to this category of ICC. The protective effect of the MS may be paradoxical regarding the higher rate of postoperative complications [48] observed in MS+ patients. We suggest that this paradox highlights the relevance of MS as prognostic factor. MS could strongly enough impact RFS despite a higher postoperative morbidity, and it does not impact significantly OS because this is not a cancer specific survival analysis. Still, the hypothesis that MS+ ICC itself carries a better prognosis should require further clinicopathological investigations.
In conclusion, the present study supports previous findings regarding the pivotal role of the MS in the development of ICC, but also confirms its impact in jeopardizing immediate postoperative results following liver resection in these patients, who should require specific attention during the perioperative period.
References
McCullough AJ (2011) Epidemiology of the metabolic syndrome in the USA. J Dig Dis 12(5):333–340
Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K et al (2005) The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med 143(10):722–728
Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ (2003) Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 348(17):1625–1638
Turati F, Talamini R, Pelucchi C, Polesel J, Franceschi S, Crispo A et al (2013) Metabolic syndrome and hepatocellular carcinoma risk. Br J Cancer 108(1):222–228
Nishioka T, Kubo S, Tanaka S, Wakasa K, Takemura S, Kinoshita M et al (2016) Outcomes of hepatic resection in intrahepatic cholangiocarcinoma patients with diabetes, hypertension, and dyslipidemia: significance of routine follow-up. Liver Cancer 5(2):107–120
Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, McGlynn KA (2011) Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatol Baltim Md 54(2):463–471
Paradis V, Zalinski S, Chelbi E, Guedj N, Degos F, Vilgrain V et al (2009) Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatol Baltim Md 49(3):851–859
Thorgeirsson SS, Grisham JW (2002) Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet 31(4):339–346
Karagozian R, Derdák Z, Baffy G (2014) Obesity-associated mechanisms of hepatocarcinogenesis. Metabolism 63(5):607–617
Cauchy F, Zalinski S, Dokmak S, Fuks D, Farges O, Castera L et al (2013) Surgical treatment of hepatocellular carcinoma associated with the metabolic syndrome. Br J Surg 100(1):113–121
Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD (2005) Cholangiocarcinoma. Lancet Lond Engl 366(9493):1303–1314
Khan SA, Toledano MB, Taylor-Robinson SD (2008) Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. HPB 10(2):77–82
Palmer WC, Patel T (2012) Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol 57(1):69–76
Kinoshita M, Kubo S, Tanaka S, Takemura S, Nishioka T, Hamano G et al (2016) The association between non-alcoholic steatohepatitis and intrahepatic cholangiocarcinoma: a hospital based case-control study. J Surg Oncol 113(7):779–783
Reddy SK, Hyder O, Marsh JW, Sotiropoulos GC, Paul A, Alexandrescu S et al (2013) Prevalence of nonalcoholic steatohepatitis among patients with resectable intrahepatic cholangiocarcinoma. J Gastrointest Surg 17(4):748–755
Viganò L, Conci S, Cescon M, Fava C, Capelli P, D’Errico A et al (2015) Liver resection for hepatocellular carcinoma in patients with metabolic syndrome: a multicenter matched analysis with HCV-related HCC. J Hepatol 63(1):93–101
Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA et al (2009) Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120(16):1640–1645
Starley BQ, Calcagno CJ, Harrison SA (2010) Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatol Baltim Md 51(5):1820–1832
Abdalla EK, Adam R, Bilchik AJ, Jaeck D, Vauthey J-N, Mahvi D (2006) Improving resectability of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol 13(10):1271–1280
Pang YY (2000) The Brisbane 2000 terminology of liver anatomy and resections. HPB 2:333–339 (HPB. 2002;4(2):99; author reply 99–100)
Jones RM, Moulton CE, Hardy KJ (1998) Central venous pressure and its effect on blood loss during liver resection. Br J Surg 85(8):1058–1060
Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D et al (2005) The «50–50 criteria» on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg 242(6):824–828 (discussion 828–829)
Ishizawa T, Hasegawa K, Kokudo N, Sano K, Imamura H, Beck Y et al (2009) Risk factors and management of ascites after liver resection to treat hepatocellular carcinoma. Arch Surg Chic Ill 1960 144(1):46–51
Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L et al (2011) Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 149(5):680–688
Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG (1992) CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Am J Infect Control 20(5):271–274
Dindo D, Demartines N, Clavien P-A (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien P-A (2013) The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 258(1):1–7
WHO classification of tumours of the digestive system, 4th edn-WHO-OMS. Disponible sur: http://apps.who.int/bookorders/anglais/detart1.jsp?codlan=1&codcol=70&codcch=4003
AJCC Cancer Staging Manual|Mahul B. Amin|Springer. Disponible sur: http://www.springer.com/us/book/9783319406176
Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW et al (2005) Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatol Baltim Md 41(6):1313–1321
Bedossa P, Poitou C, Veyrie N, Bouillot J-L, Basdevant A, Paradis V et al (2012) Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatol Baltim Md 56(5):1751–1759
Bedossa P, FLIP Pathology Consortium (2014) Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatol Baltim Md 60(2):565–575
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383
Margini C, Dufour JF (2016) The story of HCC in NAFLD: from epidemiology, across pathogenesis, to prevention and treatment. Liver Int 36(3):317–324
Wong VW-S, Wong GL-H, Yip GW-K, Lo AO-S, Limquiaco J, Chu WC-W et al (2011) Coronary artery disease and cardiovascular outcomes in patients with non-alcoholic fatty liver disease. Gut 60(12):1721–1727
Bhayani NH, Hyder O, Frederick W, Schulick RD, Wolgang CL, Hirose K et al (2012) Effect of metabolic syndrome on perioperative outcomes after liver surgery: a National Surgical Quality Improvement Program (NSQIP) analysis. Surgery 152(2):218–226
Mathur AK, Ghaferi AA, Sell K, Sonnenday CJ, Englesbe MJ, Welling TH (2010) Influence of body mass index on complications and oncologic outcomes following hepatectomy for malignancy. J Gastrointest Surg 14(5):849–857
Nobili C, Marzano E, Oussoultzoglou E, Rosso E, Addeo P, Bachellier P et al (2012) Multivariate analysis of risk factors for pulmonary complications after hepatic resection. Ann Surg 255(3):540–550
Kwok R, Choi KC, Wong GL-H, Zhang Y, Chan HL-Y, Luk AO-Y et al (2016) Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut 65(8):1359–1368
Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D et al (2008) Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg 248(1):84–96
Zhang X-F, Bagante F, Chakedis J, Moris D, Beal EW, Weiss M et al (2017) Perioperative and long-term outcome for intrahepatic cholangiocarcinoma: impact of major versus minor hepatectomy. J Gastrointest Surg 21(11):1841–1850
Cai X, Liang X, Yu T, Liang Y, Jing R, Jiang W et al (2015) Liver cirrhosis grading Child-Pugh class B: a Goliath to challenge in laparoscopic liver resection?-prior experience and matched comparisons. Hepatobiliary Surg Nutr 4(6):391–397
Han K, Ko HK, Kim KW, Won HJ, Shin YM, Kim PN (2015) Radiofrequency ablation in the treatment of unresectable intrahepatic cholangiocarcinoma: systematic review and meta-analysis. J Vasc Interv Radiol 26(7):943–948
Fujita N, Asayama Y, Nishie A, Ishigami K, Ushijima Y, Takayama Y et al (2017) Mass-forming intrahepatic cholangiocarcinoma: enhancement patterns in the arterial phase of dynamic hepatic CT—correlation with clinicopathological findings. Eur Radiol 27(2):498–506
Aishima S, Iguchi T, Nishihara Y, Fujita N, Taguchi K, Taketomi A et al (2009) Decreased intratumoral arteries reflect portal tract destruction and aggressive characteristics in intrahepatic cholangiocarcinoma. Histopathology 54(4):452–461
Bagante F, Spolverato G, Weiss M, Alexandrescu S, Marques HP, Aldrighetti L et al (2017) Assessment of the lymph node status in patients undergoing liver resection for intrahepatic cholangiocarcinoma: the new eighth edition AJCC staging system. J Gastrointest Surg 22:52–59
Türkoğlu MA, Yamamoto Y, Sugiura T, Okamura Y, Ito T, Ashida R et al (2016) The favorable prognosis after operative resection of hypervascular intrahepatic cholangiocarcinoma: a clinicopathologic and immunohistochemical study. Surgery 160(3):683–690
Spolverato G, Yakoob MY, Kim Y, Alexandrescu S, Marques HP, Lamelas J et al (2015) Impact of complications on long-term survival after resection of intrahepatic cholangiocarcinoma. Cancer 121(16):2730–2739
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any conflict of interest of any kind.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hobeika, C., Cauchy, F., Poté, N. et al. Short- and Long-Term Outcomes of Liver Resection for Intrahepatic Cholangiocarcinoma Associated with the Metabolic Syndrome. World J Surg 43, 2048–2060 (2019). https://doi.org/10.1007/s00268-019-04996-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-019-04996-y