Abstract
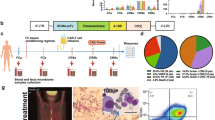
Blockade of the PD-1/PD-L1 pathway with targeted monoclonal antibodies has demonstrated encouraging anti-tumour activity in multiple cancer types. We present the case of a patient with BRAF-negative stage IVC anaplastic thyroid cancer (ATC) treated with the anti-PD-1 monoclonal antibody, pembrolizumab, following radiographic progression on chemoradiation. Blood samples were collected prior to and at four time points during treatment with pembrolizumab. Mass cytometry was used to determine expression of relevant biomarkers by peripheral blood mononuclear cells. Faecal samples were collected at baseline and 4 weeks following treatment initiation; taxonomic profiling using 16S ribosomal RNA (rRNA) gene sequencing was performed. Following treatment, a marked expansion in CD20+ B cell, CD16+ CD56lo NK cell and CD45RO+ CCR7+ central memory CD4+ T-cell populations was observed in the peripheral blood. Proportions of cells expressing the co-receptors TIGIT, OX40 and CD86 also increased during treatment. A high abundance of bacteria of the order Bacteroidales, specifically from the Bacteroidaceae and Rikenellaceae families, was identified in the faecal microbiota. Moreover, the patient’s microbiome was enriched in Clostridiales order members Ruminococcaceae, Veillonellaceae and Lachnospiraceae. Alpha diversity of the gut microbiome was significantly higher following initiation of checkpoint therapy as assessed by the Shannon and Simpson index. Our results suggest that treatment with pembrolizumab promotes expansion of T-, B- and NK cell populations in the peripheral blood at the time of tumour regression and have the potential to be implemented as predictive biomarkers in the context of checkpoint blockade therapy. Larger studies to confirm these findings are warranted.






Similar content being viewed by others
Abbreviations
- AJCC:
-
American Joint Committee on Cancer
- ATC:
-
Anaplastic thyroid cancer
- BBB:
-
Blood–brain barrier
- Bregs:
-
Regulatory B cells
- CNS:
-
Central nervous system
- CONCERT:
-
Centre for Oncology Education and Research Translation
- CT:
-
Computed tomography
- CTC:
-
Circulating tumour cells
- EBRT:
-
External beam radiation therapy
- FDG:
-
Fluorodeoxyglucose
- FNA:
-
Fine needle aspiration
- HL:
-
Hodgkin’s lymphoma
- IMRT:
-
Intensity-modulated radiation therapy
- irAEs:
-
Immune-related adverse events
- MMT:
-
Multimodal therapy
- NLR:
-
Neutrophil:lymphocyte ratio
- pDCs:
-
Plasmacytoid dendritic cells
- PET/CT:
-
Positron emission tomography/computed tomography
- RCC:
-
Renal cell carcinoma
- sOTUs:
-
Individual sequence variants
- Tfh:
-
Follicular T-helper cells
- TPS:
-
Tumour proportion score
- WBRT:
-
Whole-brain radiation therapy
References
Taccaliti A, Silvetti F, Palmonella G, Boscaro M (2012) Anaplastic thyroid carcinoma. Front Endocrinol 3:84
Cornett WR, Sharma AK, Day TA, Richardson MS, Hoda RS, van Heerden JA et al (2007) Anaplastic thyroid carcinoma: an overview. Curr Oncol Rep 9(2):152–158
Nagaiah G, Hossain A, Mooney CJ, Parmentier J, Remick SC (2011) Anaplastic thyroid cancer: a review of epidemiology, pathogenesis, and treatment. J Oncol 2011:542358
Denaro N, Nigro CL, Russi EG, Merlano MC (2013) The role of chemotherapy and latest emerging target therapies in anaplastic thyroid cancer. Oncol Targets Therp 9:1231–1241
Ayaz T, Sahin SB, Sahin OZ, Akdogan R, Gücer R (2015) Anaplastic thyroid carcinoma presenting with gastric metastasis: a case report. Hippokratia. 19(1):85–87
Besic N, Gazic B (2013) Sites of metastases of anaplastic thyroid carcinoma: autopsy findings in 45 cases from a single institution. Thyroid 23(6):709–713
Stavas MJ, Shinohara ET, Attia A, Ning MS, Friedman JM, Cmelak AJ (2014) Short course high dose radiotherapy in the treatment of anaplastic thyroid carcinoma. J Thyroid Res 2014:764281
Rao SN, Zafereo M, Dadu R, Busaidy NL, Hess K, Cote GJ et al (2017) Patterns of treatment failure in anaplastic thyroid carcinoma. Thyroid 27(5):672–681
Prasongsook N, Kumar A, Chintakuntlawar AV, Foote RL, Kasperbauer J, Molina J et al (2017) Survival in response to multimodal therapy in anaplastic thyroid cancer. J Clin Endocrinol Metab 102(12):4506–4514
Keir ME, Butte MJ, Freeman GJ, Sharpe AH (2008) PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26:677–704
Bardhan K, Anagnostou T, Boussiotis VA (2016) The PD1:PD-L1/2 pathway from discovery to clinical implementation. Front Immunol 7:550
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF et al (2012) Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med 366(26):2443–2454
Borcherding N, Kolb R, Gullicksrud J, Vikas P, Zhu Y, Zhang W (2018) Keeping tumors in check: a mechanistic review of clinical response and resistance to immune checkpoint blockade in cancer. J Mol Biol 430(14):2014–2029
Iyer PC, Dadu R, Gule-Monroe M, Busaidy NL, Ferrarotto R, Habra MA et al (2018) Salvage pembrolizumab added to kinase inhibitor therapy for the treatment of anaplastic thyroid carcinoma. J Immunotherap Cancer 6(1):68
Kollipara R, Schneider B, Radovich M, Babu S, Kiel PJ (2017) Exceptional response with immunotherapy in a patient with Anaplastic Thyroid Cancer. Oncologist 22(10):1149–1151. https://doi.org/10.1634/theoncologist.2017-0096
Wirth LJ, Eigendorff E, Capdevila J, Paz-Ares LG, Lin C-C, Taylor MH et al (2018) Phase I/II study of spartalizumab (PDR001), an anti-PD1 mAb, in patients with anaplastic thyroid cancer. J Clin Oncol 36(15_suppl):6024
Chintakuntlawar A, Yin J, Foote RL, Kasperbauer JL, Rivera M, Asmus E, Garces N, Janus J, Ma DJ, Moore EJ, Morris J, Neben-Wittich M, Price D, Ryder M, Van Abel K, Hilger CR, Samb E, Bible K (2018) A phase 2 study of pembrolizumab combined with chemoradiotherapy as initial treatment for anaplastic thyroid cancer. In: 88th Annual Meeting of the American Thyroid Association Washington, DC
Horn L, Spigel DR, Vokes EE, Holgado E, Ready N, Steins M et al (2017) Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: 2-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol 35(35):3924–3933
Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S et al (2015) Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373(19):1803–1813
Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L et al (2015) Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 372(4):320–330
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE et al (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373(17):1627–1639
Maleki Vareki S, Garrigos C, Duran I (2017) Biomarkers of response to PD-1/PD-L1 inhibition. Crit Rev Oncol Hematol 116:116–124
McGuire HM, Shklovskaya E, Edwards J, Trevillian PR, McCaughan GW, Bertolino P et al (2018) Anti-PD-1-induced high-grade hepatitis associated with corticosteroid-resistant T cells: a case report. Cancer Immunol Immunotherap CII 67(4):563–573
Imrit K, Goldfischer M, Wang J, Green J, Levine J, Lombardo J et al (2006) Identification of bacteria in formalin-fixed, paraffin-embedded heart valve tissue via 16S rRNA gene nucleotide sequencing. J Clin Microbiol 44(7):2609–2611
Handl S, Dowd SE, Garcia-Mazcorro JF, Steiner JM, Suchodolski JS (2011) Massive parallel 16S rRNA gene pyrosequencing reveals highly diverse fecal bacterial and fungal communities in healthy dogs and cats. FEMS Microbiol Ecol 76(2):301–310
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK et al (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7(5):335–336
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP (2016) DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13(7):581–583
Bokulich NA, Kaehler BD, Rideout JR, Dillon M, Bolyen E, Knight R et al (2018) Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2′s q2-feature-classifier plugin. Microbiome 6(1):90
Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, Vanderplas J (2011) Scikit-learn: machine learning in Python. J Mach Learn Res 12:2825–2830
McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A et al (2012) An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J 6(3):610–618
Li MX, Liu XM, Zhang XF, Zhang JF, Wang WL, Zhu Y et al (2014) Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. Int J Cancer 134(10):2403–2413
Paramanathan A, Saxena A, Morris DL (2014) A systematic review and meta-analysis on the impact of pre-operative neutrophil lymphocyte ratio on long term outcomes after curative intent resection of solid tumours. Surg Oncol 23(1):31–39
Templeton AJ, McNamara MG, Seruga B, Vera-Badillo FE, Aneja P, Ocana A et al (2014) Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 106(6):dju124
Ding PN, Roberts TL, Chua W, Becker TM, Descallar J, Yip PY et al (2017) Clinical outcomes in patients with advanced epidermal growth factor receptor-mutated non-small-cell lung cancer in South Western Sydney Local Health District. Int Med J 47(12):1405–1411
Sacdalan DB, Lucero JA, Sacdalan DL (2018) Prognostic utility of baseline neutrophil-to-lymphocyte ratio in patients receiving immune checkpoint inhibitors: a review and meta-analysis. OncoTargets Therap 11:955–965
Tan Q, Liu S, Liang C, Han X, Shi Y (2018) Pretreatment hematological markers predict clinical outcome in cancer patients receiving immune checkpoint inhibitors: a meta-analysis. Thoracic Cancer. 9(10):1220–1230
Cowey CL, Liu FX, Black-Shinn J, Stevinson K, Boyd M, Frytak JR et al (2018) Pembrolizumab utilization and outcomes for advanced melanoma in US community oncology practices. J Immunotherap (Hagerstown MD 1997) 41(2):86–95
Dang TO, Ogunniyi A, Barbee MS, Drilon A (2016) Pembrolizumab for the treatment of PD-L1 positive advanced or metastatic non-small cell lung cancer. Expert Rev Anticancer Ther 16(1):13–20
Fonkem E, Uhlmann EJ, Floyd SR, Mahadevan A, Kasper E, Eton O et al (2012) Melanoma brain metastasis: overview of current management and emerging targeted therapies. Expert Rev Neurother 12(10):1207–1215
Silk AW, Bassetti MF, West BT, Tsien CI, Lao CD (2013) Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med 2(6):899–906
Tallet AV, Dhermain F, Le Rhun E, Noël G, Kirova YM (2017) Combined irradiation and targeted therapy or immune checkpoint blockade in brain metastases: toxicities and efficacy. Ann Oncol 28(12):2962–2976
Long GV, Atkinson V, Lo S, Sandhu S, Guminski AD, Brown MP et al (2018) Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol 19(5):672–681
Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A et al (2018) Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol 36(28):2872–2878
Krieg C, Nowicka M, Guglietta S, Schindler S, Hartmann FJ, Weber LM et al (2018) High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat Med 24:144
Lomax AJ, McGuire HM, McNeil C, Choi CJ, Hersey P, Karikios D et al (2017) Immunotherapy-induced sarcoidosis in patients with melanoma treated with PD-1 checkpoint inhibitors: case series and immunophenotypic analysis. Int J Rheumatic Dis 20(9):1277–1285
Kamphorst AO, Pillai RN, Yang S, Nasti TH, Akondy RS, Wieland A et al (2017) Proliferation of PD-1 + CD8 T cells in peripheral blood after PD-1–targeted therapy in lung cancer patients. Proc Natl Acad Sci 114(19):4993–4998
Yuseff MI, Pierobon P, Reversat A, Lennon-Dumenil AM (2013) How B cells capture, process and present antigens: a crucial role for cell polarity. Nat Rev Immunol 13(7):475–486
Guy TV, Terry AM, Bolton HA, Hancock DG, Shklovskaya E, de Fazekas SGB (2016) Pro- and anti-tumour effects of B cells and antibodies in cancer: a comparison of clinical studies and preclinical models. Cancer Immunol Immunotherap 65(8):885–896
Varn FS, Wang Y, Cheng C (2019) A B cell-derived gene expression signature associates with an immunologically active tumor microenvironment and response to immune checkpoint blockade therapy. Oncoimmunology 8(1):e1513440
Sarvaria A, Madrigal JA, Saudemont A (2017) B cell regulation in cancer and anti-tumor immunity. Cell Mol Immunol 14(8):662–674
Grossenbacher SK, Aguilar EG, Murphy WJ (2017) Leveraging natural killer cells for cancer immunotherapy. Immunotherapy 9(6):487–497
Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K (2000) Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet (Lond Engl) 356(9244):1795–1799
Kim HS (2015) A multifaceted approach targeting NK cells for better treatment of cancer: focus on hematological malignancies. Blood Res 50(4):189–191
Armand P, Shipp MA, Ribrag V, Michot JM, Zinzani PL, Kuruvilla J et al (2016) Programmed death-1 blockade with pembrolizumab in patients with classical hodgkin lymphoma after brentuximab vedotin failure. J Clin Oncol 34(31):3733–3739
Tallerico R, Cristiani CM, Staaf E, Garofalo C, Sottile R, Capone M et al (2017) IL-15, TIM-3 and NK cells subsets predict responsiveness to anti-CTLA-4 treatment in melanoma patients. Oncoimmunology 6(2):e1261242
Labidi-Galy SI, Treilleux I, Goddard-Leon S, Combes JD, Blay JY, Ray-Coquard I et al (2012) Plasmacytoid dendritic cells infiltrating ovarian cancer are associated with poor prognosis. Oncoimmunology 1(3):380–382
Jensen TO, Schmidt H, Moller HJ, Donskov F, Hoyer M, Sjoegren P et al (2012) Intratumoral neutrophils and plasmacytoid dendritic cells indicate poor prognosis and are associated with pSTAT3 expression in AJCC stage I/II melanoma. Cancer 118(9):2476–2485
Treilleux I, Blay JY, Bendriss-Vermare N, Ray-Coquard I, Bachelot T, Guastalla JP et al (2004) Dendritic cell infiltration and prognosis of early stage breast cancer. Clin Cancer Res 10(22):7466–7474
Pinto A, Rega A, Crother TR, Sorrentino R (2012) Plasmacytoid dendritic cells and their therapeutic activity in cancer. Oncoimmunology. 1(5):726–734
Lai Y-P, Jeng C-J, Chen S-C (2011) The roles of CD4 + T cells in tumor immunity. ISRN Immunol 2011:6
Takeuchi Y, Tanemura A, Tada Y, Katayama I, Kumanogoh A, Nishikawa H (2018) Clinical response to PD-1 blockade correlates with a sub-fraction of peripheral central memory CD4 + T cells in patients with malignant melanoma. Int Immunol 30(1):13–22
Tarhini AA, Edington H, Butterfield LH, Lin Y, Shuai Y, Tawbi H et al (2014) Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PLoS One 9(2):e87705
Spitzer MH, Carmi Y, Reticker-Flynn NE, Kwek SS, Madhireddy D, Martins MM et al (2017) Systemic immunity is required for effective cancer immunotherapy. Cell 168(3):487–502.e15
Ribas A, Shin DS, Zaretsky J, Frederiksen J, Cornish A, Avramis E et al (2016) PD-1 blockade expands intratumoral memory T cells. Cancer Immunol Res 4(3):194–203
Toor SM, Syed Khaja AS, Alkurd I, Elkord E (2018) In-vitro effect of pembrolizumab on different T regulatory cell subsets. Clin Exp Immunol 191(2):189–197
Lipson EJ, Forde PM, Hammers H-J, Emens LA, Taube JM, Topalian SL (2015) Antagonists of PD-1 and PD-L1 in cancer treatment. Semin Oncol 42(4):587–600
Ott PA, Hodi FS, Kaufman HL, Wigginton JM, Wolchok JD (2017) Combination immunotherapy: a road map. J Immunother Cancer. 5:16
Li K, Qu S, Chen X, Wu Q, Shi M (2017) Promising Targets for Cancer Immunotherapy: TLRS, RLRs, and STING-mediated innate immune pathways. Int J Mol Sci 18(2):404
Kurtulus S, Sakuishi K, Ngiow SF, Joller N, Tan DJ, Teng MW et al (2015) TIGIT predominantly regulates the immune response via regulatory T cells. J Clin Investig 125(11):4053–4062
Anderson AC, Joller N, Kuchroo VK (2016) Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity 44(5):989–1004
Curti BD, Kovacsovics-Bankowski M, Morris N, Walker E, Chisholm L, Floyd K et al (2013) OX40 is a potent immune-stimulating target in late-stage cancer patients. Can Res 73(24):7189–7198
Infante JR, Hansen AR, Pishvaian MJ, Chow LQM, McArthur GA, Bauer TM et al (2016) A phase Ib dose escalation study of the OX40 agonist MOXR0916 and the PD-L1 inhibitor atezolizumab in patients with advanced solid tumors. J Clin Oncol 34(15_suppl):101
Harris SJ, Brown J, Lopez J, Yap TA (2016) Immuno-oncology combinations: raising the tail of the survival curve. Cancer Biol Med 13(2):171–193
Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R et al (2017) Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 5(1):95
Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R et al (2018) Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science (New York, NY). 359(6371):91–97
Frankel AE, Coughlin LA, Kim J, Froehlich TW, Xie Y, Frenkel EP et al (2017) Metagenomic shotgun sequencing and unbiased metabolomic profiling identify specific human gut microbiota and metabolites associated with immune checkpoint therapy efficacy in melanoma patients. Neoplasia (New York, NY). 19(10):848–855
Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C et al (2015) Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science (New York, NY). 350(6264):1079–1084
Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV et al (2018) Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science (New York, NY). 359(6371):97–103
Derbel O, Limem S, Segura-Ferlay C, Lifante JC, Carrie C, Peix JL et al (2011) Results of combined treatment of anaplastic thyroid carcinoma (ATC). BMC Cancer 11:469
Seto A, Sugitani I, Toda K, Kawabata K, Takahashi S, Saotome T (2015) Chemotherapy for anaplastic thyroid cancer using docetaxel and cisplatin: report of eight cases. Surg Today 45:221–226
Caixeiro NJ, Aghmesheh, M., de Souza P, Lee, CS (2015) The Centre for Oncology Education and Research Translation (CONCERT) Biobank. Open J Bioresour 2(1):Art. e3. doi: http://doi.org/10.5334/ojb.ai
Funding
This research was funded by the Ingham Institute for Applied Medical Research Circulating Tumour Cells (CTC) Head and Neck Research Grant, Liverpool Hospital and Cancer Council NSW (APP1147099) HM is the recipient of the Early Career Fellowship (GNT1037298). TLR is the recipient of a Cancer Institute New South Wales Future Research Leader Fellowship and salary support from Cancer Institute NSW (CINSW) translational cancer research centre CONCERT.
Author information
Authors and Affiliations
Contributions
Study conception and design was done by TLR, PS, VB, MJA, AC, HM, NN, BFSG. Experiments were performed and analysed by TLR, MJA, HM, TJ. Clinical data and samples were prepared and interpreted by TLR, AC, MJA, JS, KI. All authors made substantial contributions to data interpretation, manuscript preparation and review.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and ethical standards
All research was performed under Human Research ethics committee (HREC) protocols from Liverpool Hospital (Project number 13/097, HREC/13/LPOOL/158) (South West Sydney Local Health District), facilitated by the Centre for Oncology Education and Research Translation (CONCERT) Biobank, in accordance with relevant legislation [81].
Informed consent
Written informed consent was obtained from the patient who is the subject of the case study on January 10th, 2018. The patient agreed to the publication of this case study and the use of their specimens (provided that the participant could not be identified). Written informed consent was obtained from all controls prior to the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aghajani, M.J., Cooper, A., McGuire, H. et al. Pembrolizumab for anaplastic thyroid cancer: a case study. Cancer Immunol Immunother 68, 1921–1934 (2019). https://doi.org/10.1007/s00262-019-02416-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-019-02416-7