Abstract
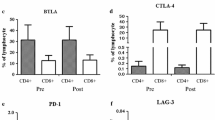
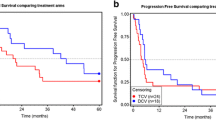
Dendritic cell (DC)-based immunotherapies have been created for a broad expanse of cancers, and DC vaccines prepared with Wilms’ tumor protein 1 (WT1) peptides have shown great therapeutic efficacy in these diseases. In this paper, we report the results of a phase I/II study of a DC-based vaccination for advanced breast, ovarian, and gastric cancers, and we offer evidence that patients can be effectively vaccinated with autologous DCs pulsed with WT1 peptide. There were ten patients who took part in this clinical study; they were treated biweekly with a WT1 peptide-pulsed DC vaccination, with toxicity and clinical and immunological responses as the principal endpoints. All of the adverse events to DC vaccinations were tolerable under an adjuvant setting. The clinical response was stable disease in seven patients. Karnofsky Performance Scale scores were enhanced, and computed tomography scans revealed tumor shrinkage in three of seven patients. Human leukocyte antigen (HLA)/WT1-tetramer and cytoplasmic IFN-γ assays were used to examine the induction of a WT-1-specific immune response. The immunological responses to DC vaccination were significantly correlated with fewer myeloid-derived suppressor cells (P = 0.045) in the pretreated peripheral blood. These outcomes offered initial clinical evidence that the WT1 peptide-pulsed DC vaccination is a potential treatment for advanced cancer.




Similar content being viewed by others
Abbreviations
- CTLs:
-
Cytotoxic T lymphocytes
- DC:
-
Dendritic cell
- ELISPOT:
-
Enzyme-linked immuno spot
- HIV:
-
Human immunodeficiency virus
- HLA:
-
Human leukocyte antigen
- IFN:
-
Interferon
- KPS:
-
Karnofsky Performance Status
- MDSCs:
-
Myeloid-derived suppressor cells
- PBMCs:
-
Peripheral blood mononuclear cells
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- TAA:
-
Tumor-associated antigens
- Treg:
-
Regulatory cells
- WT1:
-
Wilms tumor protein 1
References
Couzin-Frankel J (2013) Breakthrough of the year 2013. Cancer Immunother Sci 342:1432–1433. https://doi.org/10.1126/science.342.6165.1432
Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, Matrisian LM (2009) The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res 15:5323–5337. https://doi.org/10.1158/1078-0432.CCR-09-0737
Oji Y, Suzuki T, Nakano Y, Maruno M, Nakatsuka S, Jomgeow T, Abeno S, Tatsumi N, Yokota A, Aoyagi S, Nakazawa T, Ito K, Kanato K, Shirakata T, Nishida S, Hosen N, Kawakami M, Tsuboi A, Oka Y, Aozasa K, Yoshimine T, Sugiyama H (2004) Overexpression of the Wilms’ tumor gene WT1 in primary astrocytic tumors. Cancer Sci 95:822–827. https://doi.org/10.1111/j.1349-7006.2004.tb02188.x
Keilholz U, Letsch A, Busse A, Asemissen AM, Bauer S, Blau IW, Hofmann WK, Uharek L, Thiel E, Scheibenbogen C (2009) A clinical and immunologic phase 2 trial of Wilms tumor gene product 1 (WT1) peptide vaccination in patients with AML and MDS. Blood 113:6541–6548. https://doi.org/10.1182/blood-2009-02-202598
Tsuboi A, Oka Y, Kyo T, Katayama Y, Elisseeva OA, Kawakami M, Nishida S, Morimoto S, Murao A, Nakajima H, Hosen N, Oji Y, Sugiyama H (2012) Long-term WT1 peptide vaccination for patients with acute myeloid leukemia with minimal residual disease. Leukemia 26:1410–1413. https://doi.org/10.1038/leu.2011.343
Oka Y, Tsuboi A, Taguchi T, Osaki T, Kyo T, Nakajima H, Elisseeva OA, Oji Y, Kawakami M, Ikegame K, Hosen N, Yoshihara S, Wu F, Fujiki F, Murakami M, Masuda T, Nishida S, Shirakata T, Nakatsuka S, Sasaki A, Udaka K, Dohy H, Aozasa K, Noguchi S, Kawase I, Sugiyama H (2004) Induction of WT1 (Wilms’ tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression. Proc Natl Acad Sci USA 101:13885–13890. https://doi.org/10.1073/pnas.0405884101
Chapuis AG, Ragnarsson GB, Nguyen HN, Chaney CN, Pufnock JS, Schmitt TM, Duerkopp N, Roberts IM, Pogosov GL, Ho WY, Ochsenreither S, Wolfl M, Bar M, Radich JP, Yee C, Greenberg PD (2013) Transferred WT1-reactive CD8 + T cells can mediate antileukemic activity and persist in post-transplant patients. Sci Transl Med 5:127–174. https://doi.org/10.1126/scitranslmed.3004916
Dao T, Pankov D, Scott A, Korontsvit T, Zakhaleva V, Xu Y, Xiang J, Yan S, de Morais Guerreiro MD, Veomett N, Dubrovsky L, Curcio M, Doubrovina E, Ponomarev V, Liu C, O’Reilly RJ, Scheinberg DA (2015) Therapeutic bispecific T-cell engager antibody targeting the intracellular oncoprotein WT1. Nat Biotechnol 33:1079–1086. https://doi.org/10.1038/nbt.3349
Caldon CE, Lee CS, Sutherland RL, Musgrove EA (2008) Wilms’ tumor protein 1: an early target of progestin regulation in T-47D breast cancer cells that modulates proliferation and differentiation. Oncogene 27:126–138. https://doi.org/10.1038/sj.onc.1210622
Qi XW, Zhang F, Yang XH, Fan LJ, Zhang Y, Liang Y, Ren L, Zhong L, Chen QQ, Zhang KY, Zang WD, Wang LS, Zhang Y, Jiang J (2012) High Wilms’ tumor 1 mRNA expression correlates with basal-like and ERBB2 molecular subtypes and poor prognosis of breast cancer. Oncol Rep 28:1231–1236. https://doi.org/10.3892/or.2012.1906
Han SH, Joo M, Kim H, Chang S (2017) Mesothelin expression in gastric adenocarcinoma and its relation to clinical outcomes. J Pathol Transl Med 51:122–128. https://doi.org/10.4132/jptm.2016.11.18
Liu Z, Yamanouchi K, Ohtao T, Matsumura S, Seino M, Shridhar V, Takahashi T, Takahashi K, Kurachi H (2014) High levels of Wilms’ tumor 1 (WT1) expression were associated with aggressive clinical features in ovarian cancer. Anticancer Res 34:2331–2340
Qi XW, Zhang F, Wu H, Liu JL, Zong BG, Xu C, Jiang J (2015) Wilms’ tumor 1 (WT1) expression and prognosis in solid cancer patients: a systematic review and meta-analysis. Sci Rep 5:8924. https://doi.org/10.1038/srep08924
Noone AM, Howlader N, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds) (2018) SEER Cancer Statistics Review, 1975–2015, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2015/, based on November 2017 SEER data submission, posted to the SEER web site, April 2018
Miyatake T, Ueda Y, Morimoto A, Enomoto T, Nishida S, Shirakata T, Oka Y, Tsuboi A, Oji Y, Hosen N, Nakatsuka S, Morita S, Sakamoto J, Sugiyama H, Kimura T (2013) WT1 peptide immunotherapy for gynecologic malignancies resistant to conventional therapies: a phase II trial. J Cancer Res Clin Oncol 139:457–463. https://doi.org/10.1007/s00432-012-1348-2
Ohno S, Okuyama R, Aruga A, Sugiyama H, Yamamoto M (2012) Phase I trial of Wilms’ Tumor 1 (WT1) peptide vaccine with GM-CSF or CpG in patients with solid malignancy. Anticancer Res 32:2263–2269
Takahashi H, Okamoto M, Shimodaira S, Tsujitani S, Nagaya M, Ishidao T, Kishimoto J, Yonemitsu Y, Therapy DC-vsgatJSoIC (2013) Impact of dendritic cell vaccines pulsed with Wilms’ tumour-1 peptide antigen on the survival of patients with advanced non-small cell lung cancers. Eur J Cancer 49:852–859. https://doi.org/10.1016/j.ejca.2012.11.005
Sakai K, Shimodaira S, Maejima S, Udagawa N, Sano K, Higuchi Y, Koya T, Ochiai T, Koide M, Uehara S, Nakamura M, Sugiyama H, Yonemitsu Y, Okamoto M, Hongo K (2015) Dendritic cell-based immunotherapy targeting Wilms’ tumor 1 in patients with recurrent malignant glioma. J Neurosurg 123:989–997. https://doi.org/10.3171/2015.1.JNS141554
Palucka K, Banchereau J (2012) Cancer immunotherapy via dendritic cells. Nat Rev Cancer 12:265–277. https://doi.org/10.1038/nrc3258
Fujiki F, Oka Y, Tsuboi A, Kawakami M, Kawakatsu M, Nakajima H, Elisseeva OA, Harada Y, Ito K, Li Z, Tatsumi N, Sakaguchi N, Fujioka T, Masuda T, Yasukawa M, Udaka K, Kawase I, Oji Y, Sugiyama H (2007) Identification and characterization of a WT1 (Wilms Tumor Gene) protein-derived HLA-DRB1*0405-restricted 16-mer helper peptide that promotes the induction and activation of WT1-specific cytotoxic T lymphocytes. J Immunother 30:282–293. https://doi.org/10.1097/01.cji.0000211337.91513.94
May RJ, Dao T, Pinilla-Ibarz J, Korontsvit T, Zakhaleva V, Zhang RH, Maslak P, Scheinberg DA (2007) Peptide epitopes from the Wilms’ tumor 1 oncoprotein stimulate CD4 + and CD8 + T cells that recognize and kill human malignant mesothelioma tumor cells. Clin Cancer Res 13:4547–4555. https://doi.org/10.1158/1078-0432.CCR-07-0708
Oka Y, Tsuboi A, Elisseeva OA, Nakajima H, Fujiki F, Kawakami M, Shirakata T, Nishida S, Hosen N, Oji Y, Kawase I, Sugiyama H (2007) WT1 peptide cancer vaccine for patients with hematopoietic malignancies and solid cancers. ScientificWorldJournal 7:649–665. https://doi.org/10.1100/tsw.2007.119
Oka Y, Tsuboi A, Murakami M, Hirai M, Tominaga N, Nakajima H, Elisseeva OA, Masuda T, Nakano A, Kawakami M, Oji Y, Ikegame K, Hosen N, Udaka K, Yasukawa M, Ogawa H, Kawase I, Sugiyama H (2003) Wilms tumor gene peptide-based immunotherapy for patients with overt leukemia from myelodysplastic syndrome (MDS) or MDS with myelofibrosis. Int J Hematol 78:56–61. https://doi.org/10.1007/BF02983241
Uttenthal B, Martinez-Davila I, Ivey A, Craddock C, Chen F, Virchis A, Kottaridis P, Grimwade D, Khwaja A, Stauss H, Morris EC (2014) Wilms’ Tumour 1 (WT1) peptide vaccination in patients with acute myeloid leukaemia induces short-lived WT1-specific immune responses. Br J Haematol 164:366–375. https://doi.org/10.1111/bjh.12637
Anguille S, Van de Velde AL, Smits EL, Van Tendeloo VF, Juliusson G, Cools N, Nijs G, Stein B, Lion E, Van Driessche A, Vandenbosch I, Verlinden A, Gadisseur AP, Schroyens WA, Muylle L, Vermeulen K, Maes MB, Deiteren K, Malfait R, Gostick E, Lammens M, Couttenye MM, Jorens P, Goossens H, Price DA, Ladell K, Oka Y, Fujiki F, Oji Y, Sugiyama H, Berneman ZN (2017) Dendritic cell vaccination as postremission treatment to prevent or delay relapse in acute myeloid leukemia. Blood 130:1713–1721. https://doi.org/10.1182/blood-2017-04-780155
Fukuda K, Funakoshi T, Sakurai T, Nakamura Y, Mori M, Tanese K, Tanikawa A, Taguchi J, Fujita T, Okamoto M, Amagai M, Kawakami Y (2017) Peptide-pulsed dendritic cell vaccine in combination with carboplatin and paclitaxel chemotherapy for stage IV melanoma. Melanoma Res 27:326–334. https://doi.org/10.1097/CMR.0000000000000342
Kitawaki T, Kadowaki N, Kondo T, Ishikawa T, Ichinohe T, Teramukai S, Fukushima M, Kasai Y, Maekawa T, Uchiyama T (2008) Potential of dendritic-cell immunotherapy for relapse after allogeneic hematopoietic stem cell transplantation, shown by WT1 peptide- and keyhole-limpet-hemocyanin-pulsed, donor-derived dendritic-cell vaccine for acute myeloid leukemia. Am J Hematol 83:315–317. https://doi.org/10.1002/ajh.21127
Kobayashi M, Sakabe T, Abe H, Tanii M, Takahashi H, Chiba A, Yanagida E, Shibamoto Y, Ogasawara M, Tsujitani S, Koido S, Nagai K, Shimodaira S, Okamoto M, Yonemitsu Y, Suzuki N, Nagaya M, Therapy DC-vsgatJSoIC (2013) Dendritic cell-based immunotherapy targeting synthesized peptides for advanced biliary tract cancer. J Gastrointest Surg 17:1609–1617. https://doi.org/10.1007/s11605-013-2286-2
Koido S, Kan S, Yoshida K, Yoshizaki S, Takakura K, Namiki Y, Tsukinaga S, Odahara S, Kajihara M, Okamoto M, Ito M, Yusa S, Gong J, Sugiyama H, Ohkusa T, Homma S, Tajiri H (2014) Immunogenic modulation of cholangiocarcinoma cells by chemoimmunotherapy. Anticancer Res 34:6353–6361
Mayanagi S, Kitago M, Sakurai T, Matsuda T, Fujita T, Higuchi H, Taguchi J, Takeuchi H, Itano O, Aiura K, Hamamoto Y, Takaishi H, Okamoto M, Sunamura M, Kawakami Y, Kitagawa Y (2015) Phase I pilot study of Wilms tumor gene 1 peptide-pulsed dendritic cell vaccination combined with gemcitabine in pancreatic cancer. Cancer Sci 106:397–406. https://doi.org/10.1111/cas.12621
Saito S, Yanagisawa R, Yoshikawa K, Higuchi Y, Koya T, Yoshizawa K, Tanaka M, Sakashita K, Kobayashi T, Kurata T, Hirabayashi K, Nakazawa Y, Shiohara M, Yonemitsu Y, Okamoto M, Sugiyama H, Koike K, Shimodaira S (2015) Safety and tolerability of allogeneic dendritic cell vaccination with induction of Wilms tumor 1-specific T cells in a pediatric donor and pediatric patient with relapsed leukemia: a case report and review of the literature. Cytotherapy 17:330–335. https://doi.org/10.1016/j.jcyt.2014.10.003
Takakura K, Koido S, Kan S, Yoshida K, Mori M, Hirano Y, Ito Z, Kobayashi H, Takami S, Matsumoto Y, Kajihara M, Misawa T, Okamoto M, Sugiyama H, Homma S, Ohkusa T, Tajiri H (2015) Prognostic markers for patient outcome following vaccination with multiple MHC Class I/II-restricted WT1 peptide-pulsed dendritic cells plus chemotherapy for pancreatic cancer. Anticancer Res 35:555–562
Tsukinaga S, Kajihara M, Takakura K, Ito Z, Kanai T, Saito K, Takami S, Kobayashi H, Matsumoto Y, Odahara S, Uchiyama K, Arakawa H, Okamoto M, Sugiyama H, Sumiyama K, Ohkusa T, Koido S (2015) Prognostic significance of plasma interleukin-6/-8 in pancreatic cancer patients receiving chemoimmunotherapy. World J Gastroenterol 21:11168–11178. https://doi.org/10.3748/wjg.v21.i39.11168
Van Tendeloo VF, Van de VA, Van Driessche, Cools A, Anguille N, Ladell S, Gostick K, Vermeulen E, Pieters K, Nijs K, Stein G, Smits B, Schroyens EL, Gadisseur WA, Vrelust AP, Jorens I, Goossens PG, de Vries H, Price IJ, Oji DA, Oka Y, Sugiyama Y, Berneman H ZN (2010) Induction of complete and molecular remissions in acute myeloid leukemia by Wilms’ tumor 1 antigen-targeted dendritic cell vaccination. Proc Natl Acad Sci USA 107:13824–13829. https://doi.org/10.1073/pnas.1008051107
Kimura Y, Tsukada J, Tomoda T, Takahashi H, Imai K, Shimamura K, Sunamura M, Yonemitsu Y, Shimodaira S, Koido S, Homma S, Okamoto M (2012) Clinical and immunologic evaluation of dendritic cell-based immunotherapy in combination with gemcitabine and/or S-1 in patients with advanced pancreatic carcinoma. Pancreas 41:195–205. https://doi.org/10.1097/MPA.0b013e31822398c6
Dagvadorj N, Deuretzbacher A, Weisenberger D, Baumeister E, Trebing J, Lang I, Kochel C, Kapp M, Kapp K, Beilhack A, Hunig T, Einsele H, Wajant H, Grigoleit GU (2017) Targeting of the WT191-138 fragment to human dendritic cells improves leukemia-specific T-cell responses providing an alternative approach to WT1-based vaccination. Cancer Immunol Immunother 66:319–332. https://doi.org/10.1007/s00262-016-1938-y
Garg AD, Vara Perez M, Schaaf M, Agostinis P, Zitvogel L, Kroemer G, Galluzzi L (2017) Trial watch: dendritic cell-based anticancer immunotherapy. Oncoimmunology 6:e1328341. https://doi.org/10.1080/2162402X.2017.1328341
Wei FQ, Sun W, Wong TS, Gao W, Wen YH, Wei JW, Wei Y, Wen WP (2016) Eliciting cytotoxic T lymphocytes against human laryngeal cancer-derived antigens: evaluation of dendritic cells pulsed with a heat-treated tumor lysate and other antigen-loading strategies for dendritic-cell-based vaccination. J Exp Clin Cancer Res 35:18. https://doi.org/10.1186/s13046-016-0295-1
Ueda N, Zhang R, Tatsumi M, Liu TY, Kitayama S, Yasui Y, Sugai S, Iwama T, Senju S, Okada S, Nakatsura T, Kuzushima K, Kiyoi H, Naoe T, Kaneko S, Uemura Y (2018) BCR-ABL-specific CD4 + T-helper cells promote the priming of antigen-specific cytotoxic T cells via dendritic cells. Cell Mol Immunol 15:15–26. https://doi.org/10.1038/cmi.2016.7
Gao L, Bellantuono I, Elsasser A, Marley SB, Gordon MY, Goldman JM, Stauss HJ (2000) Selective elimination of leukemic CD34(+) progenitor cells by cytotoxic T lymphocytes specific for WT1. Blood 95:2198–2203
Nishida S, Koido S, Takeda Y, Homma S, Komita H, Takahara A, Morita S, Ito T, Morimoto S, Hara K, Tsuboi A, Oka Y, Yanagisawa S, Toyama Y, Ikegami M, Kitagawa T, Eguchi H, Wada H, Nagano H, Nakata J, Nakae Y, Hosen N, Oji Y, Tanaka T, Kawase I, Kumanogoh A, Sakamoto J, Doki Y, Mori M, Ohkusa T, Tajiri H, Sugiyama H (2014) Wilms tumor gene (WT1) peptide-based cancer vaccine combined with gemcitabine for patients with advanced pancreatic cancer. J Immunother 37:105–114. https://doi.org/10.1097/CJI.0000000000000020
Dao T, Korontsvit T, Zakhaleva V, Jarvis C, Mondello P, Oh C, Scheinberg DA (2017) An immunogenic WT1-derived peptide that induces T cell response in the context of HLA-A*02:01 and HLA-A*24:02 molecules. Oncoimmunology 6:e1252895. https://doi.org/10.1080/2162402X.2016.1252895
Tsuboi A, Oka Y, Udaka K, Murakami M, Masuda T, Nakano A, Nakajima H, Yasukawa M, Hiraki A, Oji Y, Kawakami M, Hosen N, Fujioka T, Wu F, Taniguchi Y, Nishida S, Asada M, Ogawa H, Kawase I, Sugiyama H (2002) Enhanced induction of human WT1-specific cytotoxic T lymphocytes with a 9-mer WT1 peptide modified at HLA-A*2402-binding residues. Cancer Immunol Immunother 51:614–620. https://doi.org/10.1007/s00262-002-0328-9
Koido S, Homma S, Okamoto M, Takakura K, Mori M, Yoshizaki S, Tsukinaga S, Odahara S, Koyama S, Imazu H, Uchiyama K, Kajihara M, Arakawa H, Misawa T, Toyama Y, Yanagisawa S, Ikegami M, Kan S, Hayashi K, Komita H, Kamata Y, Ito M, Ishidao T, Yusa S, Shimodaira S, Gong J, Sugiyama H, Ohkusa T, Tajiri H (2014) Treatment with chemotherapy and dendritic cells pulsed with multiple Wilms’ tumor 1 (WT1)-specific MHC class I/II-restricted epitopes for pancreatic cancer. Clin Cancer Res 20:4228–4239. https://doi.org/10.1158/1078-0432.CCR-14-0314
Shimodaira S, Sano K, Hirabayashi K, Koya T, Higuchi Y, Mizuno Y, Yamaoka N, Yuzawa M, Kobayashi T, Ito K, Koizumi T (2015) Dendritic cell-based adjuvant vaccination targeting Wilms’ tumor 1 in patients with advanced colorectal cancer. Vaccines (Basel) 3:1004–1018. https://doi.org/10.3390/vaccines3041004
Tanaka A, Sakaguchi S (2017) Regulatory T cells in cancer immunotherapy. Cell Res 27:109–118. https://doi.org/10.1038/cr.2016.151
Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC (2009) Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res 69:1553–1560. https://doi.org/10.1158/0008-5472.CAN-08-1921
Ibanez-Vea M, Zuazo M, Gato M, Arasanz H, Fernandez-Hinojal G, Escors D, Kochan G (2017) Myeloid-derived suppressor cells in the tumor microenvironment: current knowledge and future perspectives. Arch Immunol Ther Exp (Warsz). https://doi.org/10.1007/s00005-017-0492-4
Heine A, Flores C, Gevensleben H, Diehl L, Heikenwalder M, Ringelhan M, Janssen KP, Nitsche U, Garbi N, Brossart P, Knolle PA, Kurts C, Hochst B (2017) Targeting myeloid derived suppressor cells with all-trans retinoic acid is highly time-dependent in therapeutic tumor vaccination. Oncoimmunology 6:e1338995. https://doi.org/10.1080/2162402X.2017.1338995
Acknowledgements
None.
Funding
This work was supported by the National Natural Science Foundation of China (81770468), Beijing Municipal Natural Science Foundation (7162030) and the Beijing Science and Technology Plan special issue (Z14010101101).
Author information
Authors and Affiliations
Contributions
WZ, XL and PC designed and coordinated the project. CP, MX, XL, YW and XW performed the clinical and laboratory experiments. All authors take responsibility for the accuracy of the final revision of the text. LY and JL took the lead in writing the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This trial was approved by the Ethics Committee of the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, and was registered with the Chinese Clinical Trials Registry (ChiCTR-IPR-15005923).
Informed consent
Written informed consent was acquired from each patient in the study.
Rights and permissions
About this article
Cite this article
Zhang, W., Lu, X., Cui, P. et al. Phase I/II clinical trial of a Wilms’ tumor 1-targeted dendritic cell vaccination-based immunotherapy in patients with advanced cancer. Cancer Immunol Immunother 68, 121–130 (2019). https://doi.org/10.1007/s00262-018-2257-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-018-2257-2