Abstract
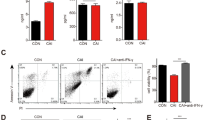
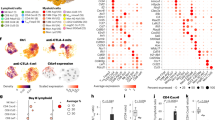
Therapeutic outcomes for adoptive cell transfer (ACT) therapy are constrained by the quality of the infused T cells. The rapid expansion necessary to obtain large numbers of cells results in a more terminally differentiated phenotype with decreased durability and functionality. N-acetyl cysteine (NAC) protects against activation-induced cell death (AICD) and improves anti-tumor efficacy of Pmel-1 T cells in vivo. Here, we show that these benefits of NAC can be extended to engineered T cells and significantly increases T-cell survival within the tumor microenvironment. The addition of NAC to the expansion protocol of human TIL13838I TCR-transduced T cells that are under evaluation in a Phase I clinical trial, demonstrated that findings in murine cells extend to human cells. Expansion of TIL13838I TCR-transduced T cells in NAC also increased their ability to kill target cells in vitro. Interestingly, NAC did not affect memory subsets, but diminished up-regulation of senescence (CD57) and exhaustion (PD-1) markers and significantly decreased expression of the transcription factors EOMES and Foxo1. Pharmacological inhibition of the PI3K/Akt pathway ablates the decrease in Foxo1 induced by NAC treatment of activated T cells. This suggests a model in which NAC through PI3K/Akt activation suppresses Foxo1 expression, thereby impacting its transcriptional targets EOMES, PD-1, and granzyme B. Taken together, our results indicate that NAC exerts pleiotropic effects that impact the quality of TCR-transduced T cells and suggest that the addition of NAC to current clinical protocols should be considered.






Similar content being viewed by others
Abbreviations
- 7-AAD:
-
7-Aminoactinomycin D
- AICD:
-
Activation-induced cell death
- EOMES:
-
Eomesodermin
- NAC:
-
N-acetyl cysteine
- REP:
-
Rapid expansion protocol
- TRP-1:
-
Tyrosinase related protein 1
References
DeSantis C, Lin C, Mariotto A et al (2014) Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 64:252–271
Rosenberg S (2012) Raising the bar: the curative potential of human cancer immunotherapy. Sci Transl Med 4:127ps8
Johnson LA, June CH (2016) Driving gene-engineered T cell immunotherapy of cancer. Cell Res 38–58
Tran KQ, Zhou J, Durflinger KH et al (2008) Minimally cultured tumor-infiltrating lymphocytes display optimal characteristics for adoptive cell therapy. J Immunother 31:742–751
Powell DJ, Dudley ME, Robbins PF, Rosenberg SA (2005) Transition of late-stage effector T cells to CD27+ CD28+ tumor-reactive effector memory T cells in humans after adoptive cell transfer therapy. Blood 105:241–250
Li Y, Liu S, Hernandez J et al (2010) MART-1-specific melanoma tumor-infiltrating lymphocytes maintaining CD28 expression have improved survival and expansion capability following antigenic restimulation in vitro. J Immunol 184:452–465
Ahmadzadeh M, Johnson LA, Heemskerk B et al (2009) Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 114:1537–1544
Hernandez-Chacon JA, Li Y, Wu RC et al (2011) Costimulation through the CD137/4-1BB pathway protects human melanoma tumor-infiltrating lymphocytes from activation-induced cell death and enhances antitumor effector function. J Immunother 34:236–250
Scheffel MJ, Scurti G, Simms P et al (2016) Efficacy of adoptive T-cell therapy is improved by treatment with the antioxidant N-acetyl cysteine, which limits activation-induced T-cell death. Cancer Res 76:6006–6016
Roszkowski JJ, Lyons GE, Kast WM et al (2005) Simultaneous generation of CD8+ and CD4+ melanoma-reactive T cells by retroviral-mediated transfer of a single T-cell receptor. Cancer Res 65:1570–1576
Norell H, Zhang Y, McCracken J et al (2010) CD34-based enrichment of genetically engineered human T cells for clinical use results in dramatically enhanced tumor targeting. Cancer Immunol Immunother 59:851–862
Overwijk WW, Theoret MR, Finkelstein SE et al (2003) Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med 198:569–580
Kerkar SP, Sanchez-Perez L, Borman Z et al (2011) Genetic engineering of murine CD8+ and CD4+ T cells for preclinical adoptive immunotherapy studies. J Immunother 34:343–352
Karlsson H, Nava S, Remberger M et al (2011) N-acetyl-l-cysteine increases acute graft-versus-host disease and promotes T-cell-mediated immunity in vitro. Eur J Immunol 41:1143–1153
Moore T, Wagner CR, Scurti GM et al (2017) Clinical and immunologic evaluation of three metastatic melanoma patients treated with autologous melanoma-reactive TCR-transduced T cells. Cancer Immunol Immunother. https://doi.org/10.1007/s00262-017-2073-0
Kesarwani P, Al-Khami AA, Scurti G et al (2014) Promoting thiol expression increases the durability of antitumor T-cell functions. Cancer Res 74:6036–6047
Klebanoff CA, Gattinoni L, Torabi-Parizi P et al (2005) Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci USA 102:9571–9576
McLane LM, Banerjee PP, Cosma GL et al (2013) Differential localization of T-bet and Eomes in CD8 T-cell memory populations. J Immunol 190:3207–3215
Pauken KE, Wherry EJ (2015) Overcoming T cell exhaustion in infection and cancer. Trends Immunol 36:265–276
Crawford A, Wherry EJ (2009) The diversity of costimulatory and inhibitory receptor pathways and the regulation of antiviral T cell responses. Curr Opin Immunol 21:179–186
Barber DL, Wherry EJ, Masopust D et al (2006) Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682–687
Staron MM, Gray SM, Marshall HD et al (2014) The transcription factor FoxO1 sustains expression of the inhibitory receptor PD-1 and survival of antiviral CD8+ T cells during chronic infection. Immunity 41:802–814
Rao RR, Li Q, Bupp MRG, Shrikant PA (2012) Transcription factor Foxo1 represses T-bet-mediated effector functions and promotes memory CD8+ T cell differentiation. Immunity 36:374–387
Aoki M, Jiang H, Vogt PK (2004) Proteasomal degradation of the FoxO1 transcriptional regulator in cells transformed by the P3k and Akt oncoproteins. Proc Natl Acad Sci USA 101:13613–13617
Zaks TZ, Chappell DB, Rosenberg SA, Restifo NP (1999) Fas-mediated suicide of tumor-reactive T cells following activation by specific tumor: selective rescue by caspase inhibition. J Immunol 162:3273–3279
Lu B, Finn OJ (2008) T-cell death and cancer immune tolerance. Cell Death Differ 15:70–79
Saff RR, Spanjaard ES, Hohlbaum AM, Marshak-Rothstein A (2004) Activation-induced cell death limits effector function of CD4 tumor-specific T cells. J Immunol 172:6598–6606
Robbins PF, Dudley ME, Wunderlich J et al (2004) Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol 173:7125–7130
Klebanoff CA, Gattinoni L, Restifo NP (2012) Sorting through subsets: which T-cell populations mediate highly effective adoptive immunotherapy? J Immunother 35:651–660
Gattinoni L, Klebanoff CA, Palmer DC et al (2005) Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest 115:1616–1626
Brenchley JM, Karandikar NJ, Betts MR et al (2003) Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8. Blood 101:2711–2720
Dudley ME, Yang JC, Sherry R et al (2008) Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol 26:5233–5239
Shen X, Zhou J, Hathcock KS et al (2007) Persistence of tumor infiltrating lymphocytes in adoptive immunotherapy correlates with telomere length. J Immunother 30:123–129
Zhou J, Shen X, Huang J et al (2005) Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol 175:7046–7052
Hsin IL, Sheu GT, Chen HH et al (2010) N-acetyl cysteine mitigates curcumin-mediated telomerase inhibition through rescuing of Sp1 reduction in A549 cells. Mutat Res 688:72–77
Liu J, Liu M, Ye X et al (2012) Delay in oocyte aging in mice by the antioxidant N-acetyl-l-cysteine (NAC). Hum Reprod 27:1411–1420
Haendeler J, Hoffmann J, Diehl JF et al (2004) Antioxidants inhibit nuclear export of telomerase reverse transcriptase and delay replicative senescence of endothelial cells. Circ Res 94:768–775
Hao LY, Strong MA, Greider CW (2004) Phosphorylation of H2AX at short telomeres in T cells and fibroblasts. J Biol Chem 279:45148–45154
Kerdiles YM, Beisner DR, Tinoco R et al (2009) Foxo1 links homing and survival of naive T cells by regulating l-selectin, CCR7 and interleukin 7 receptor. Nat Immunol 10:176–184
Matsuzaki H, Daitoku H, Hatta M et al (2003) Insulin-induced phosphorylation of FKHR (Foxo1) targets to proteasomal degradation. Proc Natl Acad Sci USA 100:11285–11290
Noh YH, Chob HS, Kim DH et al (2012) N-acetylcysteine enhances neuronal differentiation of P19 embryonic stem cells via Akt and N-cadherin activation. Mol Biol (Mosk) 46:741–746
Wang T, Mao X, Li H et al (2013) N-Acetylcysteine and allopurinol up-regulated the Jak/STAT3 and PI3K/Akt pathways via adiponectin and attenuated myocardial postischemic injury in diabetes. Free Radic Biol Med 63:291–303
Wang C, Xia Y, Zheng Y et al (2015) Protective effects of N-acetylcysteine in concanavalin a-induced hepatitis in mice. Mediators Inflamm. https://doi.org/10.1155/2015/189785
Jin HM, Zhou DC, Gu HF et al (2013) Antioxidant N-acetylcysteine protects pancreatic β-cells against aldosterone-induced oxidative stress and apoptosis in female db/db mice and insulin-producing MIN6 cells. Endocrinology 154:4068–4077
Leslie NR (2006) The redox regulation of PI 3-kinase-dependent signaling. Antioxid Redox Signal 8:1765–1774
Leslie NR, Bennett D, Lindsay YE et al (2003) Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J 22:5501–5510
Ahmad F, Nidadavolu P, Durgadoss L et al (2014) Critical cysteines in Akt1 regulate its activity and proteasomal degradation: implications for neurodegenerative diseases. Free Radic Biol Med 74:118 – 28
Kaech SM, Cui W (2012) Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol 12:749–761
Acknowledgements
The retroviral plasmid DNA encoding the MSGV-1 TRP-1 TCR was a kind gift from N. Restifo to C. Paulos.
Funding
This work was supported by the National Institutes of Health Grant P01CA154778. The Cell Evaluation and Therapy Shared Resource of the Hollings Cancer Center, Medical University of South Carolina was in part supported by the National Institutes of Health Grant P30 CA138313.
Author information
Authors and Affiliations
Contributions
MJS and CV-J conceived and designed the study. CV-J directed the project and edited the final draft of the manuscript. MJS carried out all experiments except for the aspects described for co-authors below and drafted the manuscript. GS was responsible for transduction and REP of human T cells. MMW transduced murine T cells. EG-M was responsible for the biostatistical analysis of animal experiments. GS, MMW, and EG-M drafted the corresponding method sections. CMP provided oversight for murine T-cell transduction and contributed towards the design of the study. MIN was responsible for direction and oversight of the clinical trial and contributed towards the design of the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval and ethical standards
Apheresis and cells from melanoma patients were obtained as part of clinical trial that is registered with clinicaltrials.gov NCT01586403 and was approved by the Institutional Review Board at Loyola Medical University Center (LU 203732). All procedures performed in studies using human participants were in accordance with the ethical standards of the institutional and/or national research committee, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All animal experiments were performed with approval by the Institutional Animal Care and Use Committee at the Medical University of South Carolina to ensure that ethical regulatory and policy mandates governing the use of animals in research are met (Animal Welfare Assurance #A3428-01). All methods were performed in accordance with the relevant guidelines and regulations.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Animal source
TRP-1 TCR transgenic mice, originally obtained from Dr. Nicolas Restifo, were provided by Dr. Chrystal Paulos. Recipient C57BL6 mice were purchased from Jackson Laboratories.
Cell line authentication
The tumor-cell lines in this study are used to determine T-cell reactivity and specificity, so the critical feature is their HLA and antigen expression. Cells are, therefore, routinely checked to ensure their antigen (tyrosinase) and expression HLA (HLA-A2 for MEL624) is correct.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Scheffel, M.J., Scurti, G., Wyatt, M.M. et al. N-acetyl cysteine protects anti-melanoma cytotoxic T cells from exhaustion induced by rapid expansion via the downmodulation of Foxo1 in an Akt-dependent manner. Cancer Immunol Immunother 67, 691–702 (2018). https://doi.org/10.1007/s00262-018-2120-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-018-2120-5