Abstract
Purpose
To assess the effect of gadobenate dimeglumine on magnetic resonance cholangiopancreatography (MRCP) and determine an appropriate time frame for performing MRCP sequences.
Materials and methods
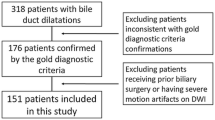
2D MRCP sequences obtained after intravenous administration of gadobenate dimeglumine or gadobutrol over 14 months were reviewed retrospectively in randomized order by five abdominal radiologists, using a 3-point scale to rate biliary and pancreatic duct clarity (1 = no-, 2 = limited-, 3 = good visualization). Intraclass correlation coefficients were computed and mean scores were compared for both agents. For gadobenate dimeglumine exams, time delays between arterial phase and MRCP acquisition times were analyzed concerning duct clarity. For gadobutrol, only exams with delays ≥ 15 min were included.
Results
134 exams (107 gadobenate dimeglumine, 27 gadobutrol) were included. Moderate reliability for pancreatic duct visualization and excellent reliability for visualization of intrahepatic bile ducts and upper and lower extrahepatic bile ducts were noted. No difference in mean scores was noted for pancreatic duct visualization (p = 0.66). Bile duct segment scores were lower with gadobenate dimeglumine (mean: 2.1–2.6) compared with gadobutrol (mean: 2.8–2.9) (p ≤ 0.006). For gadobenate dimeglumine, visualization scores varied depending on the delay between the arterial phase and MRCP acquisition (p ≤ 0.047). Good visualization for all bile duct segments was noted with delays of 7.2–9.4 min (95% confidence interval; mean 8.3 min).
Conclusion
Bile duct clarity degraded on MRCP images with an increasing delay following gadobenate dimeglumine injection. 2D MRCP, thus, should be performed within 7.2 min after obtaining the arterial phase sequence to ensure good visualization of the entire biliary system.





Similar content being viewed by others
Availability of data and material
The raw data generated and analyzed for this project have been stored and are available.
References
Patel A, Silverberg C, Becker-Weidman D, Roth C, Deshmukh S (2016) Understanding Body MRI Sequences and Their Ability to Characterize Tissues. Universal Journal of Medical Science 4(1): 1-9
Czeyda-Pommersheim F, Martin DR, Costello JR, Kalb B (2017) Contrast Agents for MR Imaging. Magnetic resonance imaging clinics of North America 25(4): 705-711
Guglielmo FF, Kania LM, Ahmad HM, Roth CG, Mitchell DG (2016) Interpreting body MRI cases: what you need to know to get started. Abdominal radiology 41(11): 2248-2269
Takahashi S, Kim T, Murakami T, Okada A, Hori M, Narumi Y, Nakamura H (2000) Influence of paramagnetic contrast on single-shot MRCP image quality. Abdominal Imaging 25(5): 511-513
Roth CG, Deshmukh S (2017) Fundamentals of Body MRI. Elsevier, Philadelphia
Koo TK, Li MY (2016) A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. Journal of Chiropractic Medicine 15(2): 155-163
Welle CL, Guglielmo FF, Venkatesh SK (2019) MRI of the liver: choosing the right contrast agent. Abdominal radiology 45: 384-392
Guglielmo FF, Mitchell DG, Gupta S (2014) Gadolinium contrast agent selection and optimal use for body MR imaging. Radiologic clinics of North America 52(4): 637-656
Frydrychowicz A, Lubner MG, Brown JJ, Merkle EM, Nagle SK, Rofsky NM, Reeder SB (2012) Hepatobiliary MR imaging with gadolinium-based contrast agents. Journal of magnetic resonance imaging 35(3): 492-511
Cavagna FM, Maggioni F, Castelli PM, Dapra M, Imperatori LG, Lorusso V, Jenkins BG (1997) Gadolinium chelates with weak binding to serum proteins. A new class of high-efficiency, general purpose contrast agents for magnetic resonance imaging. Investigative radiology 32(12): 780–796
Van Beers BE, Pastor CM, Hussain HK (2012) Primovist, Eovist: what to expect? Journal of hepatology 57(2): 421-429
Yam BL, Siegelman ES (2014) MR imaging of the biliary system. Radiologic clinics of North America 52(4): 725-755
Ringe KI, Gupta RT, Brady CM, Massey CM, Hahn A, Galanski M, Merkle EM, Lotz J (2010) Respiratory-triggered three-dimensional T2-weighted MR cholangiography after injection of gadoxetate disodium: is it still reliable? Radiology 255(2): 451-458
Yoshida M, Nakaura T, Inoue T, Tanoue S, Takada S, Utsunomiya D, Tsumagari S, Harada K, Yamashita Y. (2018) Magnetic resonance cholangiopancreatography with GRASE sequence at 3.0T: does it improve image quality and acquisition time as compared with 3D TSE?. European radiology 28(6): 2436–2443
Van Hoe L, Mermuys K, Vanhoenacker P (2004) MRCP pitfalls. Abdominal Imaging 29(3): 360-387
Griffin N, Charles-Edwards G, Grant LA (2012) Magnetic resonance cholangiopancreatography: the ABC of MRCP. Insights into imaging 3(1): 11-21
O’Connell K, Brasel K (2014) Bile metabolism and lithogenesis. The Surgical clinics of North America 94(2): 361-375
Angelin B, Bjorkhem I, Einarsson K, Ewerth S (1982) Hepatic uptake of bile acids in man. Fasting and postprandial concentrations of individual bile acids in portal venous and systemic blood serum. The Journal of clinical investigation 70(4): 724–731
Boyer JL (2013) Bile formation and secretion. Comprehensive Physiology 3(3): 1035-107
Henry AP, Attila N (2017) Bile secretion and pathophysiology of biliary tract obstruction. In: Jarnagin W (ed) Blumgart’s Surgery of the Liver, Biliary Tract and Pancreas. Elsevier, p 123
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by LMT and JKD. The first draft of the manuscript was written by LMT, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Research support/grant from Lantheus Medical Imaging, GE Healthcare, and Philips (JKD).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Trunz, L.M., Guglielmo, F.F., Selvarajan, S.K. et al. Biliary excretion of gadobenate dimeglumine causing degradation of magnetic resonance cholangiopancreatography (MRCP). Abdom Radiol 46, 562–569 (2021). https://doi.org/10.1007/s00261-020-02686-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-020-02686-1