Abstract
Purpose
PI-RADS v2 dictates that dynamic contrast-enhanced (DCE) imaging be used to further classify peripheral zone (PZ) cases that receive a diffusion-weighted imaging equivocal score of three (DWI3), a positive DCE resulting in an increase in overall assessment score to a four, indicative of clinically significant prostate cancer (csPCa). However, the accuracy of DCE in predicting csPCa in DWI3 PZ cases is unknown. This study sought to determine the frequency with which DCE changes the PI-RADS v2 DWI3 assessment category, and to determine the overall accuracy of DCE-MRI in equivocal PZ DWI3 lesions.
Materials and Methods
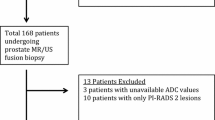
This is a retrospective study of patients with pathologically proven PCa who underwent prostate mpMRI at 3T and subsequent radical prostatectomy. PI-RADS v2 assessment categories were determined by a radiologist, aware of a diagnosis of PCa, but blinded to final pathology. csPCa was defined as a Gleason score ≥ 7 or extra prostatic extension at pathology review. Performance characteristics and diagnostic accuracy of DCE in assigning a csPCa assessment in PZ lesions were calculated.
Results
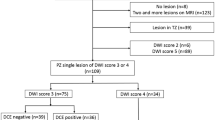
A total of 271 men with mean age of 59 ± 6 years mean PSA 6.7 ng/mL were included. csPCa was found in 212/271 (78.2%) cases at pathology, 209 of which were localized in the PZ. DCE was necessary to further classify (45/209) of patients who received a score of DWI3. DCE was positive in 29/45 cases, increasing the final PI-RADS v2 assessment category to a category 4, with 16/45 having a negative DCE. When compared with final pathology, DCE was correct in increasing the assessment category in 68.9% ± 7% (31/45) of DWI3 cases.
Conclusion
DCE increases the accuracy of detection of csPCa in the majority of PZ lesions that receive an equivocal PI-RADS v2 assessment category using DWI.


Similar content being viewed by others
References
American Cancer Society (2016) Cancer Facts & Figures 2016. Atlanta: American Cancer Society www.cancer.org. Accessed Sept 8 2018.
Hassanzadeh E, Glazer DI, Dunne RM, et al. (2017) Prostate imaging reporting and data system version 2 (PI-RADS v2): a pictorial review. Abdom Radiol 42(1):278–289
Turkbey B, Brown AM, Sankineni S, et al. (2016) Multiparametric prostate magnetic resonance imaging in the evaluation of prostate cancer. CA 66(4):326–336
Penzkofer T, Tuncali K, Fedorov A, et al. (2014) Transperineal in-bore 3-T MR imaging–guided prostate biopsy: a prospective clinical observational study. Radiology 274(1):170–180
Fennessy FM, Tuncali K, Morrison PR, Tempany CM (2010) Mr imaging–guided interventions in the genitourinary tract: an evolving concept. Magn Reson Imaging Clin 18(1):11–28
Barentsz JO, Richenberg J, Clements R, et al. (2012) ESUR prostate MR guidelines 2012. Eur Radiol 22(4):746–757
Bomers JG, Barentsz JO (2014) Standardization of multiparametric prostate MR imaging using PI-RADS. BioMed Res Int . https://doi.org/10.1155/2014/431680
Hansford BG, Peng Y, Jiang Y, et al. (2015) Dynamic contrast-enhanced MR imaging curve-type analysis: is it helpful in the differentiation of prostate cancer from healthy peripheral zone? Radiology 275(2):448–457
Platzek I, Borkowetz A, Toma M, et al. (2015) Multiparametric prostate magnetic resonance imaging at 3 T: failure of magnetic resonance spectroscopy to provide added value. J Comput Assist Tomogr 39(5):674–680
Tan CH, Paul Hobbs B, Wei W, Kundra V (2015) Dynamic contrast-enhanced MRI for the detection of prostate cancer: meta-analysis. Am J Roentgenol 204(4):W439–W448
Weinreb JC, Barentsz JO, Choyke PL, et al. (2016) PI-RADS prostate imaging–reporting and data system: 2015, version 2. Eur Urol 69(1):16–40
Park SY, Jung DC, Oh YT, et al. (2016) Prostate cancer: PI-RADS version 2 helps preoperatively predict clinically significant cancers. Radiology 280(1):108–116
Rosenkrantz AB, Verma S, Turkbey B (2015) Prostate cancer: top places where tumors hide on multiparametric MRI. Am J Roentgenol 204(4):W449–W456
Barrett T, Turkbey B, Choyke PL (2015) PI-RADS version 2: what you need to know. Clin Radiol 70(11):1165–1176
Tewes S, Mokov N, Hartung D, et al. (2016) Standardized reporting of prostate MRI: comparison of the prostate imaging reporting and data system (PI-RADS) version 1 and version 2. PLoS ONE 11(9):e0162879
Rosenkrantz AB, Babb JS, Taneja SS, Ream JM (2016) Proposed adjustments to PI-RADS version 2 decision rules: impact on prostate cancer detection. Radiology 283(1):119–129
Rosenkrantz AB, Ginocchio LA, Cornfeld D, et al. (2016) Interobserver reproducibility of the PI-RADS version 2 lexicon: a multicenter study of six experienced prostate radiologists. Radiology 280(3):793–804
Zobel BB, Quattrocchi CC, Errante Y, Grasso RF (2016) Gadolinium-based contrast agents: did we miss something in the last 25 years? Radiol Med 121(6):478–481
Idée JM, Fretellier N, Robic C, Corot C (2014) The role of gadolinium chelates in the mechanism of nephrogenic systemic fibrosis: a critical update. Crit Revs in Toxicol 44(10):895–913
Darrah TH, Prutsman-Pfeiffer JJ, Poreda RJ, et al. (2009) Incorporation of excess gadolinium into human bone from medical contrast agents. Metallomics 1(6):479–488
McDonald RJ, McDonald JS, Kallmes DF, et al. (2015) Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 275(3):772–782
Fennessy FM, Fedorov A, Penzkofer T, et al. (2015) Quantitative pharmacokinetic analysis of prostate cancer DCE-MRI at 3 T: comparison of two arterial input functions on cancer detection with digitized whole mount histopathological validation. Magn Reson Imaging 33(7):886–894
Fedorov A, Beichel R, Kalpathy-Cramer J, et al. (2012) 3D Slicer as an image computing platform for the Quantitative Imaging Network. M Magn Reson Imaging 30(9):1323–1341
Kasivisvanathan V, Rannikko AS, Borghi M, et al. (2018) MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 378(19):1767–1777
Fedorov A, Penzkofer T, Hirsch MS, et al. (2015) The role of pathology correlation approach in prostate cancer index lesion detection and quantitative analysis with multiparametric MRI. Acad Radiol 22(5):548–555
Zhang L, Tang M, Chen S, et al. (2017) A meta-analysis of use of Prostate Imaging Reporting and Data System Version 2 (PI-RADS V2) with multiparametric MR imaging for the detection of prostate cancer. Eur Radiol 27(12):5204–5214
De Visschere PI, Lumen N, Ost P, et al. (2017) Dynamic contrast-enhanced imaging has limited added value over T2-weighted imaging and diffusion-weighted imaging when using PI-RADSv2 for diagnosis of clinically significant prostate cancer in patients with elevated PSA. Clin Radiol 72(1):23–32
Epstein JI, Feng Z, Trock BJ, Pierorazio PM (2012) Upgrading and downgrading of prostate cancer from biopsy to radical prostatectomy: incidence and predictive factors using the modified Gleason grading system and factoring in tertiary grades. Eur Urol 61(5):1019–1024
D’Elia C, Cerruto MA, Cioffi A, et al. (2014) Upgrading and upstaging in prostate cancer: from prostate biopsy to radical prostatectomy. Mol Clin Oncol 2(6):1145–1149
Thestrup KC, Logager V, Baslev I, et al. (2016) Biparametric versus multiparametric MRI in the diagnosis of prostate cancer. Acta Radiol Open 5(8):2058460116663046
Fusco R, Sansone M, Petrillo M, et al. (2016) Multiparametric MRI for prostate cancer detection: preliminary results on quantitative analysis of dynamic contrast enhanced imaging, diffusion-weighted imaging and spectroscopy imaging. Magn Reson Imaging 34(7):839–845
Dinh CV, Steenbergen P, Ghobadi G, et al. (2016) Magnetic resonance imaging for prostate cancer radiotherapy. Phys Med 32(3):446–451
Barrett T, Gill AB, Kataoka MY, et al. (2012) DCE and DW MRI in monitoring response to androgen deprivation therapy in patients with prostate cancer: a feasibility study. Magn Reson Med 67(3):778–785
Panebianco V, Barchetti F, Grompone MD, et al. (2016) Magnetic resonance imaging for localization of prostate cancer in the setting of biochemical recurrence. Urol Oncol 34(7):303–310
Lista F, Gimbernat H, Cáceres F, et al. (2014) Multiparametric magnetic resonance imaging for the assessment of extracapsular invasion and other staging parameters in patients with prostate cancer candidates for radical prostatectomy. Actas Urol Esp 38(5):290–297
Kasel-Seibert M, Lehmann T, Aschenbach R, et al. (2016) Assessment of PI-RADS v2 for the detection of prostate cancer. Eur J Radiol 85(4):726–731
Vargas HA, Hötker AM, Goldman DA, et al. (2016) Updated prostate imaging reporting and data system (PIRADS v2) recommendations for the detection of clinically significant prostate cancer using multiparametric MRI: critical evaluation using whole-mount pathology as standard of reference. Eur Radiol 26(6):1606–1612
Bratan F, Melodelima C, Souchon R, et al. (2014) How accurate is multiparametric MR imaging in evaluation of prostate cancer volume? Radiology 275(1):144–154
Purysko AS, Bittencourt LK, Bullen JA, et al. (2017) Accuracy and interobserver agreement for prostate imaging reporting and data system, version 2, for the characterization of lesions identified on multiparametric MRI of the prostate. Am J Roentgenol 209(2):339–349
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Grant funding provided by U01CA151261 (FMF), P41 EB015898 (CT, FMF) and DPH403516 (FMF, CT) and R25CA89017 (FMF, AZ).
Conflicts of interest
Mehdi Taghipour declares he has no conflicts of interest. Alireza Ziaei declares he has no conflicts of interest. Elmira Hassanzadeh declares he has no conflicts of interest. Francesco Alessandrino declares he has no conflicts of interest. Mukesh Harisinghani, declares he has no conflicts of interest. Mark Vangel declares he has no conflicts of interest. Clare M Tempany received the grant NIH P41 EB015898 from the National Institute of Health – National Cancer Institute, and the grant DPH 403516 from the Massachusetts Department of Public Health. Fiona M Fennessy received the grants from the National Institute of Health – National Cancer Institute: U01 CA151261; R25 CA089017.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Taghipour, M., Ziaei, A., Alessandrino, F. et al. Investigating the role of DCE-MRI, over T2 and DWI, in accurate PI-RADS v2 assessment of clinically significant peripheral zone prostate lesions as defined at radical prostatectomy. Abdom Radiol 44, 1520–1527 (2019). https://doi.org/10.1007/s00261-018-1807-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-018-1807-6