Abstract
Purpose
To assess preoperative short-course radiotherapy (SCR) tumor response in locally advanced rectal cancer (LARC) by means of Standardized Index of Shape (SIS) by dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI), apparent diffusion coefficient (ADC), intravoxel incoherent motion (IVIM) and diffusion kurtosis imaging (DKI) parameters derived from diffusion-weighted MRI (DW-MRI).
Materials and methods
Thirty-four patients with LARC who underwent MRI scans before and after SCR followed by delayed surgery, retrospectively, were enrolled. SIS, ADC, IVIM parameters [tissue diffusion (Dt), pseudo-diffusion (Dp), perfusion fraction (fp)] and DKI parameters [mean diffusivity (MD), mean of diffusional kurtosis (MK)] were calculated for each patient. IVIM parameters were estimated using two methods, namely conventional biexponential fitting (CBFM) and variable projection (VARPRO). After surgery, the pathological TNM and tumor regression grade (TRG) were estimated. For each parameter, percentage changes between before and after SCR were evaluated. Furthermore, an artificial neural network was trained for outcome prediction. Nonparametric sample tests and receiver operating characteristic curve (ROC) analysis were performed.
Results
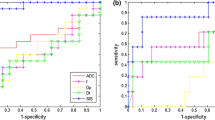
Fifteen patients were classified as responders (TRG ≤ 2) and 19 as not responders (TRG > 3). Seven patients had TRG 1 (pathological complete response, pCR). Mean and standard deviation values of pre-treatment CBFM Dp and mean value of VARPRO Dp pre-treatment showed statistically significant differences to predict pCR. (p value at Mann–Whitney test was 0.05, 0.03 and 0.008, respectively.) Exclusively SIS percentage change showed significant differences between responder and non-responder patients after SCR (p value << 0.001) and to assess pCR after SCR (p value << 0.001). The best results to predict pCR were obtained by VARPRO Fp mean value pre-treatment with area under ROC of 0.84, a sensitivity of 96.4%, a specificity of 71.4%, a positive predictive value (PPV) of 92.9%, a negative predictive value (NPV) of 83.3% and an accuracy of 91.2%. The best results to assess after treatment complete pathological response were obtained by SIS with an area under ROC of 0.89, a sensitivity of 85.7%, a specificity of 92.6%, a PPV of 75.0%, a NPV of 96.1% and an accuracy of 91.2%. Moreover, the best results to differentiate after treatment responders vs. non-responders were obtained by SIS with an area under ROC of 0.94, a sensitivity of 93.3%, a specificity of 84.2%, a PPV of 82.4%, a NPV of 94.1% and an accuracy of 88.2%. Promising initial results were obtained using a decision tree tested with all ADC, IVIM and DKI extracted parameter: we reached high accuracy to assess pathological complete response after SCR in LARC (an accuracy of 85.3% to assess pathological complete response after SCR using VARPRO Dp mean value post-treatment, ADC standard deviation value pre-treatment, MD standard deviation value post-treatment).
Conclusion
SIS is a hopeful DCE-MRI angiogenic biomarker to assess preoperative treatment response after SCR with delayed surgery. Furthermore, an important prognostic role was obtained by VARPRO Fp mean value pre-treatment and by a decision tree composed by diffusion parameters derived by DWI and DKI to assess pathological complete response.









Similar content being viewed by others
Data availability
All data were present in the text of manuscript.
Abbreviations
- AUC:
-
Area under ROC curve
- CTV:
-
Clinical target volume
- CT:
-
Computed tomography
- CBFM:
-
Conventional biexponential fitting
- DCE-MRI:
-
Dynamic contrast-enhanced magnetic resonance imaging
- DWI:
-
Diffusion-weighted imaging
- DKI:
-
Diffusion kurtosis imaging
- D t :
-
Tissue diffusion
- D p :
-
Pseudo-diffusion
- f p :
-
Perfusion fraction
- IMRT:
-
Intensity-modulated radiation therapy
- IVIM:
-
Intravoxel incoherent motion
- LARC:
-
Locally advanced rectal cancer
- MD:
-
Mean diffusivity
- MK:
-
Mean of diffusional kurtosis
- MLC:
-
Multileaf collimators
- MSD:
-
Maximum signal difference
- NPV:
-
Negative predictive value
- pCR:
-
pathological complete response
- pCRT:
-
Preoperative chemo-radiation therapy
- PPV:
-
Positive predictive value
- ROC:
-
RECEIVER operating characteristic
- ROI:
-
Regions of interest
- SCR:
-
Short-course radiotherapy
- SCRDS:
-
Short-course radiotherapy with delayed surgery
- SIS:
-
Standardized Index of Shape
- TRG:
-
Tumor regression grade
- WOS:
-
Washout slope
- VARPRO:
-
Variable projection
- VOI:
-
Volume of interest
References
Avallone A, Aloj L, Delrio P, et al. (2013) Multidisciplinary approach to rectal cancer: are we ready for selective treatment strategies? Anticancer Agents Med Chem 13(6):852–860
Avallone A, Delrio P, Guida C, et al. (2006) Biweekly oxaliplatin, raltitrexed, 5-fluorouracil and folinic acid combination chemotherapy during preoperative radiation therapy for locally advanced rectal cancer: a phase I-II study. Br J Cancer 94(12):1809–1815
Delrio P, Avallone A, Guida C, et al. (2005) Multidisciplinary approach to locally advanced rectal cancer: results of a single institution trial. Suppl Tumori. 4(3):S8.
Zhou ZR, Liu SX, Zhang TS, et al. (2014) Short-course preoperative radiotherapy with immediate surgery versus long-course chemoradiation with delayed surgery in the treatment of rectal cancer: a systematic review and meta-analysis. Surg Oncol 23(4):211–221
Latkauskas T, Pauzas H, Gineikiene I, et al. (2012) Initial results of a randomized controlled trial comparing clinical and pathological downstaging of rectal cancer after preoperative short-course radiotherapy or long-term chemoradiotherapy, both with delayed surgery. Colorectal Dis 14(3):294–298
Bujko K, Kolodziejczyk M (2008) The 5 × 5 Gy with delayed surgery in non-resectable rectal cancer: a new treatment option. Radiother Oncol 87(3):311–313
Beppu N, Matsubara N, Noda M, et al. (2015) Short-course radiotherapy with delayed surgery versus conventional chemoradiotherapy: a comparison of the short- and long-term outcomes in patients with T3 rectal cancer. Surgery 158(1):225–235.
Pettersson D, Holm T, Iversen H, et al. (2012) Preoperative short-course radiotherapy with delayed surgery in primary rectal cancer. Br J Surg 99(4):577–583
Pettersson D, Lörinc E, Holm T, et al. (2015) Tumour regression in the randomized Stockholm III trial of radiotherapy regimens for rectal cancer. Br J Surg 102(8):972–978; discussion 8.
Radu C, Berglund A, Pahlman L, Glimelius B (2008) Short-course preoperative radiotherapy with delayed surgery in rectal cancer—a retrospective study. Radiother Oncol 87(3):343–349
Hatfield P, Hingorani M, Radhakrishna G, et al. (2009) Short-course radiotherapy, with elective delay prior to surgery, in patients with unresectable rectal cancer who have poor performance status or significant co-morbidity. Radiother Oncol 92(2):210–214
Valentini V, Glimelius B, Haustermans K, et al. (2014) EURECCA consensus conference highlights about rectal cancer clinical management: the radiation oncologist’s expert review. Radiother Oncol 110(1):195–198
Avallone A, Piccirillo MC, Delrio P, et al. (2014) Phase 1/2 study of valproic acid and short-course radiotherapy plus capecitabine as preoperative treatment in low-moderate risk rectal cancer-V-shoRT-R3 (Valproic acid–short Radiotherapy–rectum 3rd trial). BMC Cancer 24(14):875
Heo SH, Kim JW, Shin SS, Jeong YY, Kang H-K (2014) Multimodal imaging evaluation in staging of rectal cancer. World J Gastroenterol 20(15):4244–4255
Fusco R, Sansone M, Petrillo M, et al. (2004) Role of magnetic resonance imaging in locally advanced rectal cancer, colorectal cancer—surgery, diagnostics and treatment. InTech. https://doi.org/10.5772/56831.
Beets-Tan RG, Beets GL (2004) Rectal cancer: review with emphasis on MR imaging. Radiology 232(2):335–346
Leach MO, Brindle KM, Evelhoch JL, et al. (2005) The assessment of antiangiogenic and antivascular therapies in early-stage clinical trials using magnetic resonance imaging: issues and recommendations. Br J Cancer 92(9):1599–1610
Petrillo A, Fusco R, Petrillo M, et al. (2015) Standardized Index of Shape (SIS): a quantitative DCE-MRI parameter to discriminate responders by non-responders after neoadjuvant therapy in LARC. Eur Radiol
Petrillo M, Fusco R, Catalano O, et al. (2015) MRI for assessing response to neoadjuvant therapy in locally advanced rectal cancer using DCE-MR and DW-MR data sets: a preliminary report. Biomed Res Int 2015:514740
Beets-Tan RG, Beets GL (2014) MRI for assessing and predicting response to neoadjuvant treatment in rectal cancer. Nat Rev Gastroenterol Hepatol 11(8):480–488
Phongkitkarun S, Tohmad U, Larbcharoensub N, et al. (2016) DCE-MRI-derived parameters as predictors of response to neo-adjuvant chemoradiation treatment of rectal carcinoma. J Med Assoc Thai. 99(3):338–347
Petrillo A, Fusco R, Granata V, et al. (2017) MR imaging perfusion and diffusion analysis to assess preoperative Short Course Radiotherapy response in locally advanced rectal cancer: Standardized Index of Shape by DCE-MRI and intravoxel incoherent motion-derived parameters by DW-MRI. Med Oncol 34(12):198
Le Bihan D, Breton E, Lallemand D, et al. (1988) Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 168(2):497–505
Le Bihan D, Breton E, Lallemand D, et al. (1986) MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 161(2):401–407
Oto A, Yang C, Kayhan A, et al. (2011) Diffusion-weighted and dynamic contrast-enhanced MRI of prostate cancer: correlation of quantitative MR parameters with Gleason score and tumor angiogenesis. AJR Am J Roentgenol 197(6):1382–1390
Curvo-Semedo L, Lambregts DM, Maas M, et al. (2011) Rectal cancer: assessment of complete response to preoperative combined radiation therapy with chemotherapy–conventional MR volumetry versus diffusion-weighted MR imaging. Radiology 260(3):734–743
Luciani A, Vignaud A, Cavet M, et al. (2008) Liver cirrhosis: intravoxel incoherent motion MR imaging-pilot study. Radiology 249(3):891–899
Wirestam R, Borg M, Brockstedt S, et al. (2001) Perfusion-related parameters in intravoxel incoherent motion MR imaging compared with CBV and CBF measured by dynamic susceptibility contrast MR technique. Acta Radiol 42(2):123–128
Granata V, Fusco R, Catalano O, et al. (2016) Intravoxel incoherent motion (IVIM) in diffusion-weighted imaging (DWI) for Hepatocellular carcinoma: correlation with histologic grade. Oncotarget 7(48):79357–79364
Granata V, Fusco R, Catalano O, et al. (2015) Early assessment of colorectal cancer patients with liver metastases treated with antiangiogenic drugs: the role of intravoxel incoherent motion in diffusion-weighted imaging. PLoS ONE 10(11):e0142876
Koh DM, Collins DJ, Orton MR (2011) Intravoxel incoherent motion in body diffusion-weighted MRI: reality and challenges. AJR Am J Roentgenol 196(6):1351–1361
Jensen JH, Helpern JA (2010) MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed 23:698–710
Dresen RC, Beets GL, Rutten HJ, et al. (2009) Locally advanced rectal cancer: MR imaging for restaging after neoadjuvant radiation therapy with concomitant chemotherapy. Part I. Are we able to predict tumor confined to the rectal wall? Radiology 252(1):81–91
SIS Tool by Antonella Petrillo available requiring at an.petrillo@istitutotumori.na.it
Fusco R, Petrillo A, Petrillo M, Sansone M (2013) Use of tracer kinetic models for selection of semi-quantitative features for DCE-MRI data classification. Appl Magn Reson 44(11):1311–1324
Fusco R, Sansone M, Petrillo A (2017) A comparison of fitting algorithms for diffusion-weighted MRI data analysis using an intravoxel incoherent motion model. MAGMA 30(2):113–120
Seber GAF, Wild CJ (1989) Nonlinear regression. New York: Wiley
Andreola S, Leo E, Belli F, et al. (2001) Adenocarcinoma of the lower third of the rectum surgically treated with a <10-mm distal clearance: preliminary results in 35 N0 patients. Ann Surg Oncol 8(7):611–615
Mandard AM, Dalibard F, Mandard JC, et al. (1994) Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 73(11):2680–2686
Fukunaga K (1990) Introduction to statistical pattern recognition. Cambridge: Academic Press
Duda RO, Hart PE, Stork DG (2001) Pattern classification. New York: Wiley
Theodoridis S, Koutroumbas K (2003) Pattern recognition. Cambridge: Academic Press
Fusco R, Sansone M, Filice S, et al. (2016) Pattern recognition approaches for breast cancer DCE-MRI classification: a systematic review. J Med Biol Eng 36(4):449–459
Choi MH, Oh SN, Rha SE, et al. (2015) Diffusion-weighted imaging: apparent diffusion coefficient histogram analysis for detecting pathologic complete response to chemoradiotherapy in locally advanced rectal cancer. J Magn Reson Imaging . https://doi.org/10.1002/jmri.25117
Doi H, Beppu N, Kato T, et al. (2015) Diffusion-weighted magnetic resonance imaging for prediction of tumor response to neoadjuvant chemoradiotherapy using irinotecan plus S-1 for rectal cancer. Mol Clin Oncol 3(5):1129–1134
Nougaret S, Vargas HA, Lakhman Y, et al. (2016) Intravoxel incoherent motion-derived histogram metrics for assessment of response after combined chemotherapy and radiation therapy in rectal cancer: initial experience and comparison between single-section and volumetric analyses. Radiology 280(2):446–454
Petrillo A, Fusco R, Petrillo M, et al. (2017) Standardized Index of Shape (DCE-MRI) and standardized uptake value (PET/CT): two quantitative approaches to discriminate chemo-radiotherapy locally advanced rectal cancer responders under a functional profile. Oncotarget 8(5):8143–8153
Iima M, Le Bihan D (2016) Clinical intravoxel incoherent motion and diffusion MR imaging: past, present, and future. Radiology 278(1):13–32
Rega D, Pecori B, Scala D, et al. (2016) Evaluation of tumor response after short-course radiotherapy and delayed surgery for rectal cancer. PLoS ONE 11(8):e0160732
Pecori B, Lastoria S, Caracò C, et al. (2017) Sequential PET/CT with [18F]-FDG predicts pathological tumor response to preoperative short course radiotherapy with delayed surgery in patients with locally advanced rectal cancer using logistic regression analysis. PLoS ONE 12(1):e0169462
Siegel R, Dresel S, Koswig S, et al. (2008) Response to preoperative short-course radiotherapy in locally advanced rectal cancer: value of f-fluorodeoxyglucose positron emission tomography. Onkologie 31(4):166–172
Janssen MH, Ollers MC, van Stiphout RG, et al. (2010) Evaluation of early metabolic responses in rectal cancer during combined radiochemotherapy or radiotherapy alone: sequential FDG-PET-CT findings. Radiother Oncol 94(2):151–155
Yu J, Xu Q, Song JC, et al. (2017) The value of diffusion kurtosis magnetic resonance imaging for assessing treatment response of neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Eur Radiol 27(5):1848–1857
Hu F, Tang W, Sun Y, et al. (2017) The value of diffusion kurtosis imaging in assessing pathological complete response to neoadjuvant chemoradiation therapy in rectal cancer: a comparison with conventional diffusion-weighted imaging. Oncotarget 8(43):75597–75606
Acknowledgements
Writing/editorial support in the preparation of this manuscript was provided by Di Giovanni Manuela, University of Technology, Sydney, Australia.
Author information
Authors and Affiliations
Contributions
All authors contributed to this work for patient’s enrollment, diagnostic and therapeutic procedures. RF performed image post-processing, statistical analysis and manuscript editing.
Corresponding author
Ethics declarations
Funding
No funding.
Conflict of interest
Each author declares that has no conflict of interest. Exclusively Robert Grimm (Robert.grimm@siemens.com) is an employee of Siemens Healthcare for development of the MR Body Diffusion Toolbox, a post-processing software to calculate IVIM and Kurtosis maps.
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Fusco, R., Sansone, M., Granata, V. et al. Diffusion and perfusion MR parameters to assess preoperative short-course radiotherapy response in locally advanced rectal cancer: a comparative explorative study among Standardized Index of Shape by DCE-MRI, intravoxel incoherent motion- and diffusion kurtosis imaging-derived parameters. Abdom Radiol 44, 3683–3700 (2019). https://doi.org/10.1007/s00261-018-1801-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-018-1801-z