Abstract
Purpose
Enlarged perivascular spaces in the centrum semiovale (CSO-EPVS) have been linked to cerebral amyloid angiopathy (CAA). To get insight into the underlying mechanisms of this association, we investigated the relationship between amyloid-β deposition assessed by 18F-florbetapir PET and CSO-EPVS in patients with acute intracerebral hemorrhage (ICH).
Methods
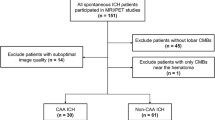
We prospectively enrolled 18 patients with lobar ICH (suggesting CAA) and 20 with deep ICH (suggesting hypertensive angiopathy), who underwent brain MRI and 18F-florbetapir PET. EPVS were assessed on MRI using a validated 4-point visual rating scale in the centrum semiovale and the basal ganglia (BG-EPVS). PET images were visually assessed, blind to clinical and MRI data. We evaluated the association between florbetapir PET positivity and high degree (score> 2) of CSO-EPVS and BG-EPVS.
Results
High CSO-EPVS degree was more common in patients with lobar ICH than deep ICH (55.6% vs. 20.0%; p = 0.02). Eight (57.1%) patients with high CSO-EPVS degree had a positive florbetapir PET compared with 4 (16.7%) with low CSO-EPVS degree (p = 0.01). In contrast, prevalence of florbetapir PET positivity was similar between patients with high vs. low BG-EPVS. In multivariable analysis adjusted for age, hypertension, and MRI markers of CAA, florbetapir PET positivity (odds ratio (OR) 6.44, 95% confidence interval (CI) 1.32–38.93; p = 0.03) was independently associated with high CSO-EPVS degree.
Conclusions
Among patients with spontaneous ICH, high degree of CSO-EPVS but not BG-EPVS is associated with amyloid PET positivity. The findings provide further evidence that CSO-EPVS are markers of vascular amyloid burden that may be useful in diagnosing CAA.


Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
References
Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344(19):1450–60.
Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9(7):689–701.
Charidimou A, Gang Q, Werring DJ. Sporadic cerebral amyloid angiopathy revisited: recent insights into pathophysiology and clinical spectrum. J Neurol Neurosurg Psychiatry. 2012;83(2):124–37.
Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 2013;12(5):483–97.
Viswanathan A, Rakich SM, Engel C, Snider R, Rosand J, Greenberg SM, et al. Antiplatelet use after intracerebral hemorrhage. Neurology. 2006;66(2):206–9.
Biffi A, Halpin A, Towfighi A, Gilson A, Busl K, Rost N, et al. Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. Neurology. 2010;75(8):693–8.
Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8(2):165–74.
Linn J, Halpin A, Demaerel P, Ruhland J, Giese AD, Dichgans M, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology. 2010;74(17):1346–50.
Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822–38.
Weller RO, Hawkes CA, Kalaria RN, Werring DJ, Carare RO. White matter changes in dementia: role of impaired drainage of interstitial fluid. Brain Pathol. 2015;25(1):63–78.
Charidimou A, Boulouis G, Pasi M, Auriel E, van Etten ES, Haley K, et al. MRI-visible perivascular spaces in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology. 2017;88(12):1157–64.
Hawkes CA, Jayakody N, Johnston DA, Bechmann I, Carare RO. Failure of perivascular drainage of beta-amyloid in cerebral amyloid angiopathy. Brain Pathol. 2014;24(4):396–403.
Gurol ME, Becker JA, Fotiadis P, Riley G, Schwab K, Johnson KA, et al. Florbetapir-PET to diagnose cerebral amyloid angiopathy: a prospective study. Neurology. 2016;87(19):2043–9.
Raposo N, Planton M, Peran P, Payoux P, Bonneville F, Lyoubi A, et al. Florbetapir imaging in cerebral amyloid angiopathy-related hemorrhages. Neurology. 2017;89(7):697–704.
Jorm AF. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): a review. Int Psychogeriatr. 2004;16(3):275–93.
Charidimou A, Linn J, Vernooij MW, Opherk C, Akoudad S, Baron JC, et al. Cortical superficial siderosis: detection and clinical significance in cerebral amyloid angiopathy and related conditions. Brain. 2015;138(Pt 8):2126–39.
Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149(2):351–6.
Doubal FN, MacLullich AM, Ferguson KJ, Dennis MS, Wardlaw JM. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke. 2010;41(3):450–4.
Maclullich AM, Wardlaw JM, Ferguson KJ, Starr JM, Seckl JR, Deary IJ. Enlarged perivascular spaces are associated with cognitive function in healthy elderly men. J Neurol Neurosurg Psychiatry. 2004;75(11):1519–23.
Johnson KA, Sperling RA, Gidicsin CM, Carmasin JS, Maye JE, Coleman RE, et al. Florbetapir (F18-AV-45) PET to assess amyloid burden in Alzheimer’s disease dementia, mild cognitive impairment, and normal aging. Alzheimers Dement. 2013;9(5 Suppl):S72–83.
Zhu YC, Tzourio C, Soumare A, Mazoyer B, Dufouil C, Chabriat H. Severity of dilated Virchow-Robin spaces is associated with age, blood pressure, and MRI markers of small vessel disease: a population-based study. Stroke. 2010;41(11):2483–90.
Banerjee G, Kim HJ, Fox Z, Jager HR, Wilson D, Charidimou A, et al. MRI-visible perivascular space location is associated with Alzheimer’s disease independently of amyloid burden. Brain. 2017;140(4):1107–16.
Martinez-Ramirez S, Pontes-Neto OM, Dumas AP, Auriel E, Halpin A, Quimby M, et al. Topography of dilated perivascular spaces in subjects from a memory clinic cohort. Neurology. 2013;80(17):1551–6.
Charidimou A, Meegahage R, Fox Z, Peeters A, Vandermeeren Y, Laloux P, et al. Enlarged perivascular spaces as a marker of underlying arteriopathy in intracerebral haemorrhage: a multicentre MRI cohort study. J Neurol Neurosurg Psychiatry. 2013;84(6):624–9.
Charidimou A, Jaunmuktane Z, Baron JC, Burnell M, Varlet P, Peeters A, et al. White matter perivascular spaces: an MRI marker in pathology-proven cerebral amyloid angiopathy? Neurology. 2014;82(1):57–62.
Charidimou A, Hong YT, Jager HR, Fox Z, Aigbirhio FI, Fryer TD, et al. White matter perivascular spaces on magnetic resonance imaging: marker of cerebrovascular amyloid burden? Stroke. 2015;46(6):1707–9.
Martinez-Ramirez S, van Rooden S, Charidimou A, van Opstal AM, Wermer M, Gurol ME, et al. Perivascular spaces volume in sporadic and hereditary (Dutch-type) cerebral amyloid angiopathy. Stroke. 2018;49(8):1913–9.
van Veluw SJ, Biessels GJ, Bouvy WH, Spliet WG, Zwanenburg JJ, Luijten PR, et al. Cerebral amyloid angiopathy severity is linked to dilation of juxtacortical perivascular spaces. J Cereb Blood Flow Metab. 2016;36(3):576–80.
Ly JV, Donnan GA, Villemagne VL, Zavala JA, Ma H, O’Keefe G, et al. 11C-PIB binding is increased in patients with cerebral amyloid angiopathy-related hemorrhage. Neurology. 2010;74(6):487–93.
Boyle PA, Yu L, Nag S, Leurgans S, Wilson RS, Bennett DA, et al. Cerebral amyloid angiopathy and cognitive outcomes in community-based older persons. Neurology. 2015;85(22):1930–6.
Acknowledgments
The authors thank the promoter of this study, CHU Toulouse.
Funding
This study was funded by Avid Radiopharmaceuticals, Toulouse Teaching Hospital (CHU) (local grant 2011 to N.R.) and the Institut des Sciences et du Cerveau de Toulouse. Avid Radiopharmaceuticals provided funding for the PET scanning and supplied the florbetapir precursor. This work has been in part supported by a grant from the French National Agency for Research called “Investissements d’Avenir” No. ANR-11-LABEX-0018-01. This work was supported by CHU Toulouse for regulatory and ethical approval and compliance. Nicolas Raposo was supported by a Fulbright Scholarship and received an Arthur Sachs Scholarship from the Harvard University Committee on General Scholarship and a Philippe Foundation research grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Payoux reports personal fees from Lilly/Avid and from GE Heathcare. Dr. Albucher received consulting fees from Bayer and speaker honoraria from Boehringer Ingelheim, Bayer, and Pfizer. Dr. Calviere received consulting fees from Boehringer Ingelheim and travel grant from Boehringer Ingelheim and Pfizer. Dr. Chollet served as a consultant for Institut de Recherche Pierre Fabre and has received speaker honoraria from Bristol-Myers Squibb and Boston Scientific. Dr. Olivot received consulting fees from AstraZeneca, Servier, and Boston Scientific and speaker honoraria from Boehringer Ingelheim, Pfizer, and Bristol-Myers Squibb. Dr. Pariente is an associate editor of the Journal of Alzheimer’s Disease and has received speaker honoraria from Lilly, Roche, and Novartis. All other authors declare that they have no conflict of interest.
Role of the funder
The funders had no role in the design or conduct of the study; in the collection, management, analysis, or interpretation of the data; in the preparation, review, or approval of the manuscript; or in the decision to submit the manuscript for publication.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee (Toulouse-Purpan Hospital Ethical Standards Committee on Human Experimentation; No. 1122302) and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from each participant (or a legally authorized representative) included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurology
Rights and permissions
About this article
Cite this article
Raposo, N., Planton, M., Payoux, P. et al. Enlarged perivascular spaces and florbetapir uptake in patients with intracerebral hemorrhage. Eur J Nucl Med Mol Imaging 46, 2339–2347 (2019). https://doi.org/10.1007/s00259-019-04441-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04441-1