Abstract
Purpose
Our purpose in this study was to assess the added clinical value of hybrid 18F–FDG-PET/MRI compared to conventional imaging for locoregional staging in breast cancer patients undergoing neoadjuvant chemotherapy (NAC).
Methods
In this prospective study, primary invasive cT2-4 N0 or cT1-4 N+ breast cancer patients undergoing NAC were included. A PET/MRI breast protocol was performed before treatment. MR images were evaluated by a breast radiologist, blinded for PET images. PET images were evaluated by a nuclear physician. Afterwards, a combined PET/MRI report was written. PET/MRI staging was compared to conventional imaging, i.e., mammography, ultrasound and MRI. The proportion of patients with a modified treatment plan based on PET/MRI findings was analyzed.
Results
A total of 40 patients was included. PET/MRI was of added clinical value in 20.0% (8/40) of patients, changing the treatment plan in 10% and confirming the malignancy of suspicious lesions on MRI in another 10%. In seven (17.5%) patients radiotherapy fields were extended because of additional or affirmative PET/MRI findings being lymph node metastases (n = 5) and sternal bone metastases (n = 2). In one (2.5%) patient radiotherapy fields were reduced because of fewer lymph node metastases on PET/MRI compared to conventional imaging. Interestingly, all treatment changes were based on differences in number of lymph nodes suspicious for metastasis or number of distant metastasis, whereas differences in intramammary tumor extent were not observed.
Conclusion
Prior to NAC, PET/MRI shows promising results for locoregional staging compared to conventional imaging, changing the treatment plan in 10% of patients and potentially replacing PET/CT or tissue sampling in another 10% of patients.
Similar content being viewed by others
Introduction
Accurate locoregional staging prior to neoadjuvant chemo- and immunotherapy (NAC) in breast cancer patients is important to determine prognosis and to define an individual surgical and radiotherapy treatment plan after NAC. Standard staging imaging at breast cancer diagnosis is performed with full-field digital mammography (FFDM) and ultrasound (US). In a neoadjuvant setting, various international guidelines recommend magnetic resonance imaging (MRI) to monitor response to treatment [1,2,3].
Locoregional staging prior to NAC (pre-NAC) remains challenging. Primary tumor diameter often differs between imaging modalities. Studies have proven MRI to be the most accurate modality for measuring tumor extent, particularly in a neoadjuvant setting [3,4,5,6]. The number and location of lymph node metastases, which are important indicators for clinical decision-making and determining locoregional recurrence (LRR) risk, cannot be assessed adequately prior to NAC. Schipper et al. have shown that US cannot accurately assess the number of lymph node metastases, with a reported NPV of just 50% to differentiate between one and three and four or more axillary lymph node metastases [7].
Previous studies have shown fluorodeoxyglucose (18F–FDG) positron emission tomography (PET)/ computed tomography (CT) is of added value in nodal staging [8,9,10,11,12,13,14,15]. PET/CT shows lymph node metastases in the internal mammary chain (IMC) and periclavicular area that are not detected on conventional imaging. The number of axillary lymph nodes suspicious for metastases is often higher on PET/CT compared to conventional imaging [8,9,10,11]. The specificity of PET/CT for axillary lymph node metastases is around 96%, compared to about 78% on US and breast MRI [16,17,18].
Since MRI is most suitable for soft tissue imaging, like breast and possible lymph node morphology, and PET has the advantage of showing increased metabolic uptake in lymph node (and distant) metastases, a combined approach in the form of hybrid PET/MRI may potentially provide improved locoregional breast cancer staging. With this complementary diagnostic information, both breast and nodal status could be determined more accurately prior to NAC within a single scan.
The purpose of this study was to assess the added clinical value of hybrid 18F–FDG PET/MRI (PET/MRI) compared to conventional imaging (i.e. FFDM, US and MRI) for locoregional staging prior to NAC in breast cancer patients.
Materials and methods
Patient selection and study design
This prospective single center study was approved by the institutional review board. Informed consent was waived by the institutional review board. Women with biopsy-proven primary invasive breast cancer with a tumor larger than 2 cm (cT2-4 N0) and/or a pathologically confirmed lymph node metastasis (cT1-4 N+) undergoing NAC between February 2015 and June 2016 were consecutively considered for inclusion [19]. Exclusion criteria were pregnancy, presence of distant metastases at diagnosis or contra-indications for PET/MR imaging (such as known allergies for the contrast agents used or severe claustrophobia).
Conventional pre-NAC imaging consisted of FFDM and US of the suspicious breast lesion(s) and ipsilateral axilla. Breast cancer diagnosis and initial cTNM-classification were based on conventional imaging combined with pathology of pre-treatment core needle biopsies. If suspicious axillary lymph nodes were visualized, US-guided core needle biopsy of the most suspicious lymph node was performed. Reports of all conventional imaging exams and pathology reports were written in accordance with the Dutch Breast Cancer Guidelines [3].
After the decision to start NAC by a multidisciplinary tumor board in a newly diagnosed breast cancer patient, a PET/MRI breast protocol was performed prior to treatment initiation. Results concerning clinical tumor status (cN) and clinical nodal status and metastatic status (cN) on PET/MRI were compared to cT and cNstatus based on FFDM, US and MRI (the MR images made with PET/MRI were first interpreted separately, blinded for PET images). The percentage of patients with a modified treatment plan based on PET/MRI findings was analyzed.
Hybrid 18F–FDG PET/MRI protocol
Blood glucose levels had to be <10 mmol/l after a fasting period of at least 4 h. All PET/MR images were obtained after intravenous injection of a bodyweight adapted 18F–FDG dose (2 MBq/kg bodyweight) followed by a resting period of 45–60 min. All scans were performed from diaphragm to top of the humeral head on the same 3.0 T Biograph mMR integrated PET/MRI system (Siemens Healthcare, Erlangen, Germany) using a dedicated bilateral 16-channel breast radiofrequency coil (Rapid Biomedical, Rimpar, Germany) while patients were placed in a prone position and with both arms above their head.
The MR imaging protocol consisted of a two-dimensional T2-weighted turbo spin-echo sequence without fat suppression, a diffusion weighted imaging (DWI) sequence with fat suppression and a dynamic contrast enhanced T1-weighted sequence with fat suppression. As contrast agent, Gadobutrol (Gadovist®, Bayer Health Care, Germany) was used. It was automatically injected through a catheter in the antecubital vein at a 0.1 mmol/kg bodyweight, followed by a saline flush.
Parameters used for T2-weighted imaging consisted of a 340 mm field of view (FOV), a voxel size of 0.9 × 0.8 × 3.0 mm, 46 slices, 6410 milliseconds repetition time (TR), 83 milliseconds echo time (TE), 5 min 28 s acquisition time, turbo factor 11 and 80 degrees flip angle in transverse plane. For DWI a 320 mm FOV, voxel size of 1.7 × 1.7 × 4.0 mm and 24 slices were used and b-values 50, 400, 800 and 1000 s/mm2 were acquired. For T1-weighted imaging, a 340 mm FOV, 0.9 × 0.9 × 1.2 mm voxel size, 128 slices, 10 degrees flip angle, 4.77 milliseconds TR, 1.78 milliseconds TE and 9.02 min acquisition time for a normal size breast were used. Magnetic resonance images were assessed by a dedicated breast radiologist with seven years of experience, blinded for PET results, using the descriptors of the American College of Radiology MRI BI-RADS lexicon [20].
The PET scanner has an axial FOV of 258 mm. All PET images were iteratively reconstructed and automatically attenuation corrected with the implemented 4-compartment model attenuation map (μ-map). All PET images (one bed position) were acquired within 11 min of the initial activity measurement. PET images were evaluated by a dedicated nuclear medicine physician with four years of experience. A lesion was characterized as malignant if it showed a focally increased FDG uptake compared to the surrounding breast tissue. After initial separate assessment, both imaging specialists performed a consensus reading of both PET and MRI images and a combined conclusion was drawn.
Imaging analysis and staging
To determine clinical tumor (cT) stage on conventional imaging, number of breast lesions (unifocal or multifocal) and size (largest diameter of the largest malignant breast lesion in one view) were measured on FFDM and US. For clinical nodal (cN), staging number and location of suspicious lymph nodes were determined on US. Characteristics of a suspicious axillary lymph node on US included diffuse cortical thickening, focal cortical mass and/or thickening and loss of the fatty hilum [21].
To determine cT-stage on MRI, also a number of breast lesions and tumor size were assessed on MRI. To determine size on MRI, the largest diameter of the largest breast lesion proven to be malignant was measured on the T1-weighted MRI sequence at peek enhancement (i.e., first dynamic phase after contrast injection) in one view. For determining cN-stage, number and location of suspicious lymph nodes were assessed on MRI. The following criteria were considered suspicious: irregular margins, inhomogeneous cortex, perifocal edema, absent fatty hilum, asymmetry, and absence of chemical shift artifacts [22,23,24].
To determine cT-stage on PET/MRI, the diameter of the tumor was measured on MRI, as described above, as diameters are not clinically measured on PET. Therefore, tumor size was always the same on MRI and PET/MRI. Yet, uni- or multifocality was determined on both modalities (pathologically proven malignant hotspot on PET or pathologically proven suspicious lesion on MRI). For determining cN-stage, number and location of suspicious lymph nodes were assessed on both modalities. A lymph node was characterized as malignant on PET if an abnormal focal FDG accumulation was present in combination with high visual uptake intensity (VUI) compared to background tissue, as recommended by the European Association of Nuclear Medicine [17, 25].
If any distant metastasis was detected in the FOV of any imaging modality (a lesion outside the breast tissue and lymph nodes like a bone or lung) and also proven PET positive and/or pathologically confirmed malignant, patients were considered M1, if not they were considered M0.
Treatment
Neoadjuvant chemotherapy generally consisted of four cycles of 3-weekly doxorubicin and cyclofosfamide, followed by four cycles of 3-weekly docetaxel in case of an ER+ and/or HER2 overexpressed tumor, or 12 cycles of weekly paclitaxel in case of a triple negative tumor. In patients with HER2 overexpressed tumors, trastuzumab was added. After NAC, breast conserving surgery (BCS) or mastectomy and surgery of the ipsilateral axilla (sentinel lymph node biopsy in case of N0 and axillary lymph node dissection in case of N+) was performed. Postoperative radiotherapy of the breast was always performed after BCS. Chest wall and periclavicular irradiation were performed when patients had a cN1 (≥4 suspicious nodes), cT1-2ypN+, c/ypT3N+, c/ypT4 or c/ypN2–3 status. Solitary chest wall irradiation was performed in case of irradical breast surgery. The internal mammary chain (IMC) was irradiated in case of a PET-positive or a pathologically proven tumor-positive lymph node in IMC.
Results
A total of 40 women with primary invasive breast cancer treated with NAC were consecutively included. One patient had to be excluded because she did not fit into the scanner due to her size. All PET/MR images were acquired before initiation of NAC and 4–28 days (median 10 days) after primary diagnostic conventional imaging and biopsy procedures. Baseline characteristics are shown in Table 1. In eight out of 40 patients (20%, of which two distant metastases) PET/MRI was of added clinical value. In four out of 40 patients clinically relevant lesions (one distant metastasis) found on PET/MRI, but not on MRI or conventional imaging, lead to treatment plan changes (Table 2 and Appendix Table 3). In another four out of 40 patients PET/MRI confirmed malignancy of suspicious lesions on MRI (one distant metastasis), thereby potentially replacing PET/CT or tissue sampling.
cT-stage: Breast tumor size and focality
On conventional imaging, mean tumor size was 33 mm and one patient had a multifocal tumor. After MR imaging, four patients had a multifocal tumor (all pathologically proven) and mean tumor size on MRI was 37 mm. Hybrid PET/MRI did not find additional multifocal tumors meaning that clinical tumor stage, based on the size of the primary tumor measured only on MRI (not on PET) and number of breast lesions (multifocality, measured on both MRI and PET), did not differ from MRI alone (Table 2 and Appendix Table 3). Hence, PET/MRI did not provide diagnostic advantages compared to MRI alone for breast tumor staging, nor did it result in treatment plan changes.
cN-stage: Number and location of suspicious lymph nodes
According to conventional imaging, 12 out of 40 patients were considered cN0 and 28 patients cN+. In six out of 40 patients, lymph node status changed based on PET/MRI findings. In these six patients number and location of lymph node hotspots on PET, combined with their morphology on MRI, resulted in confirmation of diagnosis and change in treatment plan.
In three patients, MRI showed an enlarged IMC node. PET/MRI confirmed malignancy of this node by showing focally enhanced FDG-uptake in the IMC node in these patients, making additional PET/CT imaging or tissue sampling superfluous. In another patient a suspicious IMC node with focally enhanced FDG-uptake was seen. No suspicious IMC node was described on MRI at first. The final combination of PET information with MRI morphology completed diagnosis and changed the treatment plan. In all four patients the IMC was incorporated in the radiotherapy field.
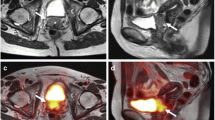
One patient had five axillary FDG hotspots suspicious for lymph node metastases on PET/MRI, whereas initially only two were seen on US (of which one was proven to be malignant by tissue sampling) and no lymph nodes suspicious for metastases were described on MRI alone. Combining PET information with MRI, all five lymph nodes were marked as suspicious for metastases. As a consequence, chest wall and periclavicular area were added to the radiotherapy field. Image examples are shown in Fig. 1.
Images of a patient with no lymph nodes suspicious for metastases on MRI (T2w sequence is shown in the left column) and five axillary FDG hotspots suspicious for lymph node metastases on PET (small arrows, middle column). Adding PET information to MRI, resulted in five lymph nodes marked as suspicious for metastases (big arrows, right column)
One patient had three axillary lymph nodes suspicious for metastases on PET/MRI compared to more than three on US and MRI. Final clinical decision by the multidisciplinary tumor board was to consider three lymph nodes to be suspicious for metastases. The chest wall and periclavicular area were, therefore, excluded from the radiotherapy field. Images are shown in Fig. 2.
Additional findings – Distant metastases
PET/MRI confirmed and changed metastatic status in two patients. In both cases a sternal bone metastasis was found. In one patient a sternal bone abnormality was detected by the radiologist on MRI. PET/MRI confirmed malignancy by showing focally enhanced FDG-uptake of this bone abnormality, making additional PET/CT imaging or tissue sampling superfluous. In the other patient the bone lesion was not described in the MRI report at first. In both cases a hotspot on PET combined with morphologic information on MRI completed diagnosis. In retrospect, the bone metastasis of the second patient was visible as a subtle abnormality on T2-weighted MR images. In both patients the treatment plan was adjusted. In both cases a whole body PET/CT was acquired consecutively and did not show other distant metastases. Both patients are being treated curatively (oligometastatic breast cancer), and the sternal bone will be additionally irradiated.
Discussion
Accurate pre-NAC staging in breast cancer patients is important as it reflects prognosis and determines treatment plan after NAC. This study demonstrated the added clinical value of hybrid PET/MRI compared to conventional imaging and MRI for locoregional staging prior to NAC in breast cancer patients. For tumor staging, PET/MRI was not of added value compared to MRI alone. However, in 10% of patients PET/MRI detected nodal or distant metastases, which were not detected on MRI or conventional imaging modalities. In another 10% it confirmed malignancy of lesions characterized as probably malignant on MRI, making additional PET/CT imaging or tissue sampling redundant. The treatment plan was adjusted in all these patients.
Considering cT-stage, a recent study by Grueneisen et al. found that both PET/MRI and MRI enable better determination of breast tumor extent in comparison to PET/CT [26]. Similar to our results, PET/MRI did not provide diagnostic advantages for breast tumor staging compared to MRI alone. These results underline the importance of breast MRI for primary tumor staging of breast cancer patients.
Regarding cN-stage, Grueneisen et al. looked at axillary lymph node involvement. PET/MRI, MRI and PET/CT allowed for a correct positive or negative axillary nodal status in 86%, 80% and 88%, respectively (p > 0,05) [26]. Similar to our results, PET shows best results for axillary lymph node staging. On the contrary, Taneja and colleagues showed a lower sensitivity on PET (60%) than on MRI (93.3%) for detection of axillary lymph nodes with PET/MRI [27]. A possible explanation for the lower PET detection rate is that PET/MRI scans in the study of Taneja might have been made during NAC treatment (this was not specified). Since previous research demonstrated that PET shows response to NAC treatment earlier than MRI [28], a PET/MRI scan made during NAC treatment might no longer show lymph node metastases on PET, due to early metabolic response, while remaining suspicious on MRI.
Next to differentiating between a positive or negative axillary nodal status, our study showed the importance of evaluating the exact number of positive axillary lymph nodes and the involvement of extra-axillary lymph nodes (IMC and periclavicular area) with PET/MRI. The pre-NAC presence of lymph nodes suspicious for metastases in the IMC, axilla (>3 tumor-positive nodes) or periclavicular area are important determinants for the extent of radiotherapy fields and for risk of LRR. This cannot be accurately determined pre-NAC with conventional staging techniques, as described by Schipper et al. [7], or in post-NAC surgery specimens due to pre-treatment.
This study suggests that approximately 1 in 13 patients treated with NAC may harbor undetected lymph node metastases (including axillary, periclavicular and IMC nodes) when they do not undergo a pre-NAC PET(/MRI) scan. Patients with undetected lymph nodes in the IMC or periclavicular area treated with conventional tangential fields in case of BCS, only receive low scatter doses in these areas, leaving these nodes (partially) untreated.
Extended nodal US imaging could be considered an alternative to PET/MRI. However, US imaging of the IMC is labor-intensive, operator dependent and no hard evidence favoring it can be found in the literature [11, 29]. Furthermore, research has shown US is not suited to differentiate between >3 or ≤3 axillary tumor-positive axillary nodes and that it is body composition dependent [7]. Finally, PET/MRI can show focally enhanced FDG uptake in morphologically normal lymph nodes. PET/MRI may, therefore, be superior for locoregional N-staging, potentially changing prognosis and treatment plan.
Finally, all included patients were M0 at diagnosis. Our results suggest that 2/40 patients treated with NAC have sternal bone metastases, of which 1/40 would remain undetected when they do not undergo a pre-NAC PET(/MRI) scan. Studies from the 1980’s indicate that 1.9–2.4% of breast cancer patients have solitary sternal bone metastases, compared to 5% in our study [30, 31]. The latter might be higher due to the higher risk population with patients receiving NAC in our study. Nevertheless, similar to the undetected lymph node metastases, these sternal bone metastases will only receive low radiotherapy scatter doses in case of BCS or chest wall irradiation, leaving them (partially) untreated.
Since current guidelines advise additional PET(/CT) imaging or tissue sampling for suspicious lymph/metastatic nodes on MRI or conventional imaging, PET/MRI functioned as a diagnostic confirmation tool instead of PET/CT or tissue sampling in 10% of our patients. For example, all IMC nodes were also visible on MRI (one in retrospect). Future research focusing on the MRI characteristics determining malignancy of metastatic (IMC) nodes may replace PET imaging or tissue sampling. The value of IMC radiation in terms of breast cancer recurrence and (cardio)toxicity remains debatable. As Aukema et al. suggested, a patient-tailored radiotherapy field around the PET-positive node instead of the whole IMC might be a solution in the future.
One of the limitations of our prospective study is its small sample size. If selection bias occurred, our small sample size may over- or underestimate the magnitude of the added value of PET/MRI for locoregional staging prior to NAC. Since patients were included consecutively, risk for selection bias was minimized. Furthermore, as patients in this study were treated with NAC, axillary surgery was performed after neoadjuvant treatment. A pathologist cannot reliably determine the pre-NAC number of lymph node metastases in a post-NAC surgical sample [32]. We do know that PET specificity for axillary lymph nodes is 94% [17]. Therefore, it is safe to assume that the PET-positive nodes were tumor-positive and patients were correctly upstaged [11].
In conclusion, pre-NAC hybrid PET/MRI shows promising results for locoregional breast cancer staging when compared to FFDM, US and MRI alone. For staging the primary tumor, PET/MRI is equally as good as MRI. For locoregional N and M-staging, PET/MRI is of added clinical value, detecting nodal and distant metastases not detected as such on MRI or conventional imaging modalities and thereby changing the treatment plan in 10% of patients. In another 10% of patients, PET/MRI made additional PET/CT imaging or tissue sampling superfluous by confirming malignancy of suspicious lesions on MRI.
References
Cardoso F, Costa A, Senkus E, Aapro M, Andre F, Barrios CH, et al. 3rd ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 3). Ann Oncol. 2016; doi:10.1093/annonc/mdw544.
American College of Radiology (ACR). ACR Practice parameter for the performance of contrastenhanced magnetic resonance Imaging (MRI) of the breast. 2014. Available from: http://www.acr.org.
Nationaal Borstkanker Overleg Nederland (NABON). Richtlijn Mammacarcinoom, versie 2.0 (Breast Cancer Dutch Guideline, version 2.0). 2012. Available from: http://www.oncoline.nl/uploaded/docs/mammacarcinoom/Dutch%20Breast%20Cancer%20Guideline%202012.pdf.
Pediconi F, Miglio E, Telesca M, Luciani ML, Kirchin MA, Passariello R, et al. Effect of preoperative breast magnetic resonance imaging on surgical decision making and cancer recurrence rates. Investig Radiol. 2012;47:128–35. doi:10.1097/RLI.0b013e318230061c.
Gruber IV, Rueckert M, Kagan KO, Staebler A, Siegmann KC, Hartkopf A, et al. Measurement of tumour size with mammography, sonography and magnetic resonance imaging as compared to histological tumour size in primary breast cancer. BMC Cancer. 2013;13:328. doi:10.1186/1471-2407-13-328.
Lobbes MB, Prevos R, Smidt M, Tjan-Heijnen VC, van Goethem M, Schipper R, et al. The role of magnetic resonance imaging in assessing residual disease and pathologic complete response in breast cancer patients receiving neoadjuvant chemotherapy: a systematic review. Insights Imaging. 2013;4:163–75. doi:10.1007/s13244-013-0219-y.
Schipper RJ, van Roozendaal LM, de Vries B, Pijnappel RM, Beets-Tan RG, Lobbes MB, et al. Axillary ultrasound for preoperative nodal staging in breast cancer patients: is it of added value? Breast. 2013;22:1108–13. doi:10.1016/j.breast.2013.09.002.
Koolen BB, Valdes Olmos RA, Vogel WV, Vrancken Peeters MJ, Rodenhuis S, Rutgers EJ, et al. Pre-chemotherapy 18F-FDG PET/CT upstages nodal stage in stage II-III breast cancer patients treated with neoadjuvant chemotherapy. Breast Cancer res Treat. 2013;141:249–54. doi:10.1007/s10549-013-2678-8.
Krammer J, Schnitzer A, Kaiser CG, Buesing KA, Sperk E, Brade J, et al. (18) F-FDG PET/CT for initial staging in breast cancer patients - is there a relevant impact on treatment planning compared to conventional staging modalities? Eur Radiol. 2015;25:2460–9. doi:10.1007/s00330-015-3630-6.
Liu Y. Role of FDG PET-CT in evaluation of locoregional nodal disease for initial staging of breast cancer. World J Clin Oncol. 2014;5:982–9. doi:10.5306/wjco.v5.i5.982.
Aukema TS, Straver ME, Peeters MJ, Russell NS, Gilhuijs KG, Vogel WV, et al. Detection of extra-axillary lymph node involvement with FDG PET/CT in patients with stage II-III breast cancer. Eur J Cancer. 2010;46:3205–10. doi:10.1016/j.ejca.2010.07.034.
Koolen BB, Valdes Olmos RA, Elkhuizen PH, Vogel WV, Vrancken Peeters MJ, Rodenhuis S, et al. Locoregional lymph node involvement on 18F-FDG PET/CT in breast cancer patients scheduled for neoadjuvant chemotherapy. Breast Cancer res Treat. 2012;135:231–40. doi:10.1007/s10549-012-2179-1.
Choi YJ, Shin YD, Kang YH, Lee MS, Lee MK, Cho BS, et al. The effects of preoperative (18)F-FDG PET/CT in breast cancer patients in comparison to the conventional imaging study. J Breast Cancer. 2012;15:441–8. doi:10.4048/jbc.2012.15.4.441.
Ng SP, David S, Alamgeer M, Ganju V. Impact of pretreatment combined (18)F-Fluorodeoxyglucose positron emission tomography/computed tomography staging on radiation therapy treatment decisions in locally advanced breast cancer. Int J Radiat Oncol Biol Phys. 2015;93:111–7. doi:10.1016/j.ijrobp.2015.05.012.
Segaert I, Mottaghy F, Ceyssens S, De Wever W, Stroobants S, Van Ongeval C, et al. Additional value of PET-CT in staging of clinical stage IIB and III breast cancer. Breast J. 2010;16:617–24. doi:10.1111/j.1524-4741.2010.00987.x.
van Nijnatten TJ, Ploumen EH, Schipper RJ, Goorts B, Andriessen EH, Vanwetswinkel S, et al. Routine use of standard breast MRI compared to axillary ultrasound for differentiating between no, limited and advanced axillary nodal disease in newly diagnosed breast cancer patients. Eur J Radiol. 2016;85:2288–94. doi:10.1016/j.ejrad.2016.10.030.
Cooper KL, Harnan S, Meng Y, Ward SE, Fitzgerald P, Papaioannou D, et al. Positron emission tomography (PET) for assessment of axillary lymph node status in early breast cancer: a systematic review and meta-analysis. Eur J Surg Oncol. 2011;37:187–98. doi:10.1016/j.ejso.2011.01.003.
An YS, Lee DH, Yoon JK, Lee SJ, Kim TH, Kang DK, et al. Diagnostic performance of 18F-FDG PET/CT, ultrasonography and MRI. Detection of axillary lymph node metastasis in breast cancer patients. Nuklearmedizin. 2014;53:89–94. doi:10.3413/Nukmed-0605-13-06.
The Union for International Cancer Control (UICC). TNM classification of malignant tumors. 1999. Wiley Blackwell. Available from: http://www.uicc.org/resources/tnm.
Morris EA, Comstock CE, Lee CH, et al. ACR BI-RADS® magnetic resonance imaging. ACR BI-RADS® atlas, breast imaging reporting and data system. Reston: American College of Radiology. 2013. Available from: https://www.acr.org/Quality-Safety/Resources/BIRADS/MRI.
Neal CH, Daly CP, Nees AV, Helvie MA. Can preoperative axillary US help exclude N2 and N3 metastatic breast cancer? Radiology. 2010;257:335–41. doi:10.1148/radiol.10100296.
Baltzer PA, Dietzel M, Burmeister HP, Zoubi R, Gajda M, Camara O, et al. Application of MR mammography beyond local staging: is there a potential to accurately assess axillary lymph nodes? Evaluation of an extended protocol in an initial prospective study. AJR am J Roentgenol. 2011;196:W641–7. doi:10.2214/AJR.10.4889.
Farshchian N, Tamari S, Farshchian N, Madani H, Rezaie M, Mohammadi-Motlagh HR. Diagnostic value of chemical shift artifact in distinguishing benign lymphadenopathy. Eur J Radiol. 2011;80:594–7. doi:10.1016/j.ejrad.2010.10.005.
Lambregts DM, Heijnen LA, Maas M, Rutten IJ, Martens MH, Backes WH, et al. Gadofosveset-enhanced MRI for the assessment of rectal cancer lymph nodes: predictive criteria. Abdom Imaging. 2013;38:720–7. doi:10.1007/s00261-012-9957-4.
Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl med Mol Imaging. 2015;42:328–54. doi:10.1007/s00259-014-2961-x.
Grueneisen J, Nagarajah J, Buchbender C, Hoffmann O, Schaarschmidt BM, Poeppel T, et al. Positron emission tomography/magnetic resonance imaging for local tumor staging in patients with primary breast cancer: a comparison with positron emission tomography/computed tomography and magnetic resonance imaging. Investig Radiol. 2015;50:505–13. doi:10.1097/RLI.0000000000000197.
Taneja S, Jena A, Goel R, Sarin R, Kaul S. Simultaneous whole-body (1)(8)F-FDG PET-MRI in primary staging of breast cancer: a pilot study. Eur J Radiol. 2014;83:2231–9. doi:10.1016/j.ejrad.2014.09.008.
Groheux D, Mankoff D, Espie M, Hindie E. (1)(8)F-FDG PET/CT in the early prediction of pathological response in aggressive subtypes of breast cancer: review of the literature and recommendations for use in clinical trials. Eur J Nucl med Mol Imaging. 2016;43:983–93. doi:10.1007/s00259-015-3295-z.
Ozdemir H, Atilla S, Ilgit ET, Isik S. Parasternal sonography of the internal mammary lymphatics in breast cancer: CT correlation. Eur J Radiol. 1995;19:114–7.
Noguchi S, Miyauchi K, Nishizawa Y, Imaoka S, Koyama H, Iwanaga T. Results of surgical treatment for sternal metastasis of breast cancer. Cancer. 1988;62:1397–401.
Park HM, Tarver RD. Solitary sternal metastasis from breast carcinoma. Clin Nucl med. 1983;8:373–4.
Bossuyt V, Symmans WF. Standardizing of pathology in patients receiving neoadjuvant chemotherapy. Ann Surg Oncol. 2016;23:3153–61. doi:10.1245/s10434-016-5317-x.
Acknowledgements
The authors thank the dedicated PET/MRI radiographers Eslina Selanno, Kim Brouwers, Rianda Cobben and Renee Franssen and the entire breast cancer treatment team of the Maastricht University Medical Center for their support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by an academic incentive from the Maastricht University Medical Center + .
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments.
Informed consent
Informed consent was waived by the institutional research committee.
Financial support
Academic incentive from the Maastricht University Medical Center+.
Appendix
Appendix
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Goorts, B., Vöö, S., van Nijnatten, T.J.A. et al. Hybrid 18F–FDG PET/MRI might improve locoregional staging of breast cancer patients prior to neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging 44, 1796–1805 (2017). https://doi.org/10.1007/s00259-017-3745-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-017-3745-x