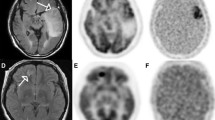
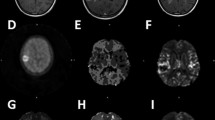
Abstract
Purpose
Hypoxia in gliomas is associated with tumor resistance to radio- and chemotherapy. However, positron emission tomography (PET) imaging of hypoxia remains challenging, and the validation of biological markers is, therefore, of great importance. We investigated the relationship between uptake of the PET hypoxia tracer [18F]-FMISO and other markers of hypoxia and angiogenesis and with patient survival.
Patients and methods
In this prospective single center clinical study, 33 glioma patients (grade IV: n = 24, III: n = 3, and II: n = 6) underwent [18F]-FMISO PET and MRI including relative cerebral blood volume (rCBV) maps before surgery. Maximum standardized uptake values (SUVmax) and hypoxic volume were calculated, defining two groups of patients based on the presence or absence of [18F]-FMISO uptake. After surgery, molecular quantification of CAIX, VEGF, Ang2 (rt-qPCR), and HIF-1α (immunohistochemistry) were performed on tumor specimens.
Results
[18F]-FMISO PET uptake was closely linked to tumor grade, with high uptake in glioblastomas (GB, grade IV). Expression of biomarkers of hypoxia (CAIX, HIF-1α), and angiogenesis markers (VEGF, Ang2, rCBV) were significantly higher in the [18F]-FMISO uptake group. We found correlations between the degree of hypoxia (hypoxic volume and SUVmax) and expression of HIF-1α, CAIX, VEGF, Ang2, and rCBV (p < 0.01). Patients without [18F]-FMISO uptake had a longer survival time than uptake positive patients (log-rank, p < 0.005).
Conclusions
Tumor hypoxia as evaluated by [18F]-FMISO PET is associated with the expression of hypoxia markers on a molecular level and is related to angiogenesis. [18F]-FMISO uptake is a mark of an aggressive tumor, almost always a glioblastoma. Our results underline that [18F]-FMISO PET could be useful to guide glioma treatment, and in particular radiotherapy, since hypoxia is a well-known factor of resistance.






Similar content being viewed by others
References
Rampling R, Cruickshank G, Lewis AD, Fitzsimmons SA, Workman P. Direct measurement of pO2 distribution and bioreductive enzymes in human malignant brain tumors. Int J Radiat Oncol Biol Phys. 1994;29(3):427–31.
Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109.
Evans SM, Judy KD, Dunphy I, et al. Hypoxia is important in the biology and aggression of human glial brain tumors. Clin Cancer Res. 2004;10(24):8177–84.
Evans SM, Jenkins KW, Chen HI, et al. The relationship among hypoxia, proliferation, and outcome in patients with de novo glioblastoma: a pilot study. Transl Oncol. 2010;3(3):160–9.
Szeto MD, Chakraborty G, Hadley J, et al. Quantitative metrics of net proliferation and invasion link biological aggressiveness assessed by MRI with hypoxia assessed by FMISO-PET in newly diagnosed glioblastomas. Cancer Res. 2009;69(10):4502–9.
Brown JM. Therapeutic targets in radiotherapy. Int J Radiat Oncol Biol Phys. 2001;49(2):319–26.
Hall EJ, Giaccia AJ. Radiobiology for the radiologist. 7th ed. Philadelphia: LWW; 2011. 576p.
Flynn JR, Wang L, Gillespie DL, et al. Hypoxia-regulated protein expression, patient characteristics, and preoperative imaging as predictors of survival in adults with glioblastoma multiforme. Cancer. 2008;113(5):1032–42.
Spence AM, Muzi M, Swanson KR, et al. Regional hypoxia in glioblastoma multiforme quantified with [18F]fluoromisonidazole positron emission tomography before radiotherapy: correlation with time to progression and survival. Clin Cancer Res. 2008;14(9):2623–30.
Corroyer-Dulmont A, Chakhoyan A, Collet S, et al. Imaging modalities to assess oxygen status in glioblastoma. Front Med. 2015. doi:10.3389/fmed.2015.00057.
Chen W, Silverman DHS. Advances in evaluation of primary brain tumors. Semin Nucl Med. 2008;38(4):240–50.
Derlon JM, Chapon F, Noël MH, et al. Non-invasive grading of oligodendrogliomas: correlation between in vivo metabolic pattern and histopathology. Eur J Nucl Med. 2000;27(7):778–87.
Collet S, Valable S, Constans JM, et al. [(18)F]-fluoro-L-thymidine PET and advanced MRI for preoperative grading of gliomas. Neuroimage Clin. 2015;8:448–54.
Valk PE, Mathis CA, Prados MD, Gilbert JC, Budinger TF. Hypoxia in human gliomas: demonstration by PET with fluorine-18-fluoromisonidazole. J Nucl Med. 1992;33:2133–7.
Cher LM, Murone C, Lawrentschuk N, et al. Correlation of hypoxic cell fraction and angiogenesis with glucose metabolic rate in gliomas using 18F-fluoromisonidazole, 18F-FDG PET, and immunohistochemical studies. J Nucl Med. 2006;47(3):410–8.
Lee ST, Scott AM. Hypoxia positron emission tomography imaging with 18f-fluoromisonidazole. Semin Nucl Med. 2007;37(6):451–61.
Koh WJ, Rasey JS, Evans ML, et al. Imaging of hypoxia in human tumors with [F-18]fluoromisonidazole. Int J Radiat Oncol Biol Phys. 1992;22(1):199–212.
Rasey JS, Koh WJ, Evans ML, et al. Quantifying regional hypoxia in human tumors with positron emission tomography of [18F]fluoromisonidazole: a pretherapy study of 37 patients. Int J Radiat Oncol Biol Phys. 1996;36(2):417–28.
Bernsen HJ, Rijken PF, Peters H, et al. Hypoxia in a human intracerebral glioma model. J Neurosurg. 2000;93(3):449–54.
Sharp FR, Bernaudin M. HIF1 and oxygen sensing in the brain. Nat Rev Neurosci. 2004;5(6):437–48.
Harris AL. Hypoxia, a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2(1):38–47.
Denko NC, Fontana LA, Hudson KM, et al. Investigating hypoxic tumor physiology through gene expression patterns. Oncogene. 2003;22(37):5907–14.
Said HM, Hagemann C, Staab A, et al. Expression patterns of the hypoxia-related genes osteopontin, CA9, erythropoietin, VEGF and HIF-1alpha in human glioma in vitro and in vivo. Radiother Oncol. 2007;83(3):398–405.
Pastorek J, Pastorekova S. Hypoxia-induced carbonic anhydrase IX as a target for cancer therapy: from biology to clinical use. Semin Cancer Biol. 2015;31:52–64.
Fagiani E, Christofori G. Angiopoietins in angiogenesis. Cancer Lett. 2013;328(1):18–26.
Bruehlmeier M, Roelcke U, Schubiger PA, Ametamey SM. Assessment of hypoxia and perfusion in human brain tumors using PET with 18F-fluoromisonidazole and 15O-H2O. J Nucl Med. 2004;45(11):1851–9.
Yamamoto Y, Maeda Y, Kawai N, et al. Hypoxia assessed by 18F-fluoromisonidazole positron emission tomography in newly diagnosed gliomas. Nucl Med Commun. 2012;33(6):621–5.
Kawai N, Lin W, Cao W-D, et al. Correlation between 18F-fluoromisonidazole PET and expression of HIF-1α and VEGF in newly diagnosed and recurrent malignant gliomas. Eur J Nucl Med Mol Imaging. 2014;41(10):1870–8.
Chang CW, Chou TK, Liu RS, et al. A robotic synthesis of [18F]fluoromisonidazole ([18F]FMISO). Appl Radiat Isot. 2007;65(6):682–6.
Valable S, Petit E, Roussel S, et al. Complementary information from magnetic resonance imaging and (18)F-fluoromisonidazole positron emission tomography in the assessment of the response to an antiangiogenic treatment in a rat brain tumor model. Nucl Med Biol. 2011;38(6):781–93.
Swanson KR, Chakraborty G, Wang CH, et al. Complementary but distinct roles for MRI and 18F-Fluoromisonidazole PET in the assessment of human glioblastomas. J Nucl Med. 2009;50(1):36–44.
Yasui H, Matsumoto S, Devasahayam N, et al. Low-field magnetic resonance imaging to visualize chronic and cycling hypoxia in tumor-bearing mice. Cancer Res. 2010;70(16):6427–36.
Gerstner ER, Zhang Z, Fink JR, et al. ACRIN 6684: assessment of tumor hypoxia in newly diagnosed glioblastoma using 18F-FMISO PET and MRI. Clin Cancer Res. 2016;22(20):5079–86.
Vartanian A, Singh SK, Agnihotri S, et al. GBM’s multifaceted landscape: highlighting regional and microenvironmental heterogeneity. Neuro-Oncology. 2014;16(9):1167–75.
Bell C, Dowson N, Fay M, et al. Hypoxia imaging in gliomas with 18F-fluoromisonidazole PET: toward clinical translation. Semin Nucl Med. 2015;45(2):136–50.
Hirata K, Terasaka S, Shiga T, et al. 18F-Fluoromisonidazole positron emission tomography may differentiate glioblastoma multiforme from less malignant gliomas. Eur J Nucl Med Mol Imaging. 2012;39(5):760–70.
Toyonaga T, Yamaguchi S, Hirata K, et al. Hypoxic glucose metabolism in glioblastoma as a potential prognostic factor. Eur J Nucl Med Mol Imaging. 2016. doi:10.1371/journal.pone.0167917.
Acknowledgements
We thank neurosurgeons S. Khoury, B. Gadan, A. Borha. We also thank S. de Bouard, J. Pierre, S. Lecot-Cotigny and N. Elie. We thank V. Constans and S. Kabani for editing the manuscript.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This study was funded by Centre National de la Recherche Scientifique (CNRS), Université de Caen Normandie (UCN), Conseil Régional de Basse-Normandie (CRBN) and Institut National du Cancer (INCa).
Conflict of interest
None of the authors have a conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Bekaert, L., Valable, S., Lechapt-Zalcman, E. et al. [18F]-FMISO PET study of hypoxia in gliomas before surgery: correlation with molecular markers of hypoxia and angiogenesis. Eur J Nucl Med Mol Imaging 44, 1383–1392 (2017). https://doi.org/10.1007/s00259-017-3677-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-017-3677-5