Abstract
Purpose
We investigated dual-phase 18F-florbetapir (AV-45/Amyvid) PET imaging for the concomitant detection of brain perfusion deficits and beta-amyloid deposition in patients with Alzheimer’s disease (AD) and amnestic mild cognitive impairment (MCI), and in cognitively healthy controls (HCs).
Methods
A total of 82 subjects (24 AD patients, 44 MCI patients and 14 HCs) underwent both dual-phase 18F-AV-45 PET and MRI imaging. Dual-phase dynamic PET imaging consisted of (1) five 1-min scans obtained 1 – 6 min after tracer injection (perfusion 18F-AV-45 imaging, pAV-45), and (2) ten 1-min scans obtained 50 – 60 min after tracer injection (amyloid 18F-AV-45 imaging). Amyloid-negative MCI/AD patients were excluded. Volume of interest analysis and statistical parametric mapping of pAV-45 and 18F-AV-45 images were performed to investigate the perfusion deficits and the beta-amyloid burden in the three study groups. The associations between Mini-Mental State Examination (MMSE) scores and global perfusion deficits and amyloid deposition were investigated with linear and segmental linear correlation analyses.
Results
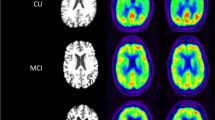
HCs generally had normal pAV-45 findings, whereas perfusion deficits were evident in the hippocampus, and temporal, parietal and middle frontal cortices in both MCI and AD patients. The motor-sensory cortex was relatively preserved. MMSE scores in the entire study cohort were significantly associated with the degree of perfusion impairment as assessed by pAV-45 imaging (r = 0.5156, P < 0.0001). 18F-AV-45 uptake was significantly higher in AD patients than in the two other study groups. However, the correlation between MMSE scores and 18F-AV-45 uptake in MCI patients was more of a binary phenomenon and began in MCI patients with MMSE score 23.14 when 18F-AV-45 uptake was higher and MMSE score lower than in patients with early MCI. Amyloid deposition started in the precuneus and the frontal and temporal regions in early MCI, ultimately reaching the maximum burden in advanced MCI.
Conclusion
Our results indicate that brain perfusion deficits and beta-amyloid deposition in AD follow different trajectories that can be successfully traced using dual-phase 18F-AV-45 PET imaging.





Similar content being viewed by others
References
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–9.
Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–19.
Lin KJ, Hsu WC, Hsiao IT, Wey SP, Jin LW, Skovronsky D, et al. Whole-body biodistribution and brain PET imaging with [18F]AV-45, a novel amyloid imaging agent – a pilot study. Nucl Med Biol. 2010;37:497–508.
Nelissen N, Van Laere K, Thurfjell L, Owenius R, Vandenbulcke M, Koole M, et al. Phase 1 study of the Pittsburgh compound B derivative 18F-flutemetamol in healthy volunteers and patients with probable Alzheimer disease. J Nucl Med. 2009;50:1251–9.
Rowe CC, Ackerman U, Browne W, Mulligan R, Pike KL, O'Keefe G, et al. Imaging of amyloid beta in Alzheimer's disease with 18F-BAY94-9172, a novel PET tracer: proof of mechanism. Lancet Neurol. 2008;7:129–35.
Jagust WJ, Friedland RP, Budinger TF. Positron emission tomography with [18F]fluorodeoxyglucose differentiates normal pressure hydrocephalus from Alzheimer-type dementia. J Neurol Neurosurg Psychiatry. 1985;48:1091–6.
Smith FW, Besson JA, Gemmell HG, Sharp PF. The use of technetium-99m-HM-PAO in the assessment of patients with dementia and other neuropsychiatric conditions. J Cereb Blood Flow Metab. 1988;8:S116–22.
Jack CR, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–28.
Landau SM, Mintun MA, Joshi AD, Koeppe RA, Petersen RC, Aisen PS, et al. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol. 2012;72:578–86.
Wu L, Rowley J, Mohades S, Leuzy A, Dauar MT, Shin M, et al. Dissociation between brain amyloid deposition and metabolism in early mild cognitive impairment. PLoS One. 2012;7:e47905.
Hsiao IT, Huang CC, Hsieh CJ, Hsu WC, Wey SP, Yen TC, et al. Correlation of early-phase 18F-florbetapir (AV-45/Amyvid) PET images to FDG images: preliminary studies. Eur J Nucl Med Mol Imaging. 2012;39:613–20.
Meyer PT, Hellwig S, Amtage F, Rottenburger C, Sahm U, Reuland P, et al. Dual-biomarker imaging of regional cerebral amyloid load and neuronal activity in dementia with PET and 11C-labeled Pittsburgh compound B. J Nucl Med. 2011;52:393–400.
Koeppe RA, Gilman S, Joshi A, Liu S, Little R, Junck L, et al. 11C-DTBZ and 18F-FDG PET measures in differentiating dementias. J Nucl Med. 2005;46:936–44.
Treyer V, Streffer J, Wyss MT, Bettio A, Ametamey SM, Fischer U, et al. Evaluation of the metabotropic glutamate receptor subtype 5 using PET and 11C-ABP688: assessment of methods. J Nucl Med. 2007;48:1207–15.
Farid K, Hong YT, Aigbirhio FI, Fryer TD, Menon DK, Warburton EA, et al. Early-phase 11C-PiB PET in amyloid angiopathy-related symptomatic cerebral hemorrhage: potential diagnostic value? PLoS One. 2015;10:e0139926.
Gur RC, Ragland JD, Reivich M, Greenberg JH, Alavi A, Gur RE. Regional differences in the coupling between resting cerebral blood flow and metabolism may indicate action preparedness as a default state. Cereb Cortex. 2009;19:375–82.
Murase K, Tanada S, Fujita H, Sakaki S, Hamamoto K. Kinetic behavior of technetium-99m-HMPAO in the human brain and quantification of cerebral blood flow using dynamic SPECT. J Nucl Med. 1992;33:135–43.
Wong CY, Thie J, Gaskill M, Ponto R, Hill J, Tian HY, et al. A statistical investigation of normal regional intra-subject heterogeneity of brain metabolism and perfusion by F-18 FDG and O-15 H2O PET imaging. BMC Nucl Med. 2006;6:4.
Chen Y, Wolk D, Reddin J, Korczykowski M, Martinez P, Musiek E, et al. Voxel-level comparison of arterial spin-labeled perfusion MRI and FDG-PET in Alzheimer disease. Neurology. 2011;77:1977–85.
Musiek ES, Chen Y, Korczykowski M, Saboury B, Martinez PM, Reddin JS, et al. Direct comparison of fluorodeoxyglucose positron emission tomography and arterial spin labeling magnetic resonance imaging in Alzheimer's disease. Alzheimers Dement. 2012;8:51–9.
Rostomian AH, Madison C, Rabinovici GD, Jagust WJ. Early [11C]PIB frames and [18F]FDG PET measures are comparable: a study validated in a cohort of AD and FTLD patients. J Nucl Med. 2011;52:173–9.
Huang KL, Lin KJ, Hsiao IT, Kuo HC, Hsu WC, Chuang WL, et al. Regional amyloid deposition in amnestic mild cognitive impairment and Alzheimer's disease evaluated by [18F]AV-45 positron emission tomography in Chinese population. PLoS One. 2013;8:e58974.
Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment – beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–6.
Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–92.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders, DSM-IV. Washington, DC: American Psychiatric Association.
Lin RT, Lai CL, Tai CT, Liu CK, Yen YY, Howng SL. Prevalence and subtypes of dementia in southern Taiwan: impact of age, sex, education, and urbanization. J Neurol Sci. 1998;160:67–75.
Liu CK, Lin RT, Chen YF, Tai CT, Yen YY, Howng SL. Prevalence of dementia in an urban area in Taiwan. J Formos Med Assoc. 1996;95:762–8.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–44.
Yao CH, Lin KJ, Weng CC, Hsiao IT, Ting YS, Yen TC, et al. GMP-compliant automated synthesis of [(18)F]AV-45 (Florbetapir F18) for imaging beta-amyloid plaques in human brain. Appl Radiat Isot. 2010;68:2293–7.
Xia M, Wang J, He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One. 2013;8:e68910.
Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J. A probabilistic atlas of the human brain: theory and rationale for its development. The International Consortium for Brain Mapping (ICBM). Neuroimage. 1995;2:89–101.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89.
Pagani M, De Carli F, Morbelli S, Öberg J, Chincarini A, Frisoni G, et al. Volume of interest-based [18F]fluorodeoxyglucose PET discriminates MCI converting to Alzheimer's disease from healthy controls. A European Alzheimer's Disease Consortium (EADC) study. NeuroImage Clin. 2015;7:34–42.
Clark CM, Schneider JA, Bedell BJ, Beach TG, Bilker WB, Mintun MA, et al. Use of florbetapir-PET for imaging β-amyloid pathology. JAMA. 2011;305:275–83.
Johnson KA, Sperling RA, Gidicsin CM, Carmasin JS, Maye JE, Coleman RE, et al. Florbetapir (F18-AV-45) PET to assess amyloid burden in Alzheimer's disease dementia, mild cognitive impairment, and normal aging. Alzheimers Dement. 2013;9:S72–83.
Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299–309.
Del Sole A, Clerici F, Chiti A, Lecchi M, Mariani C, Maggiore L, et al. Individual cerebral metabolic deficits in Alzheimer’s disease and amnestic mild cognitive impairment: an FDG PET study. Eur J Nucl Med Mol Imaging. 2008;35:1357–66.
Silverman DH, Alavi A. PET imaging in the assessment of normal and impaired cognitive function. Radiol Clin North Am. 2005;43:67–77.
Habeck C, Risacher S, Lee GJ, Glymour MM, Mormino E, Mukherjee S, et al. Relationship between baseline brain metabolism measured using [18F]FDG PET and memory and executive function in prodromal and early Alzheimer’s disease. Brain Imaging Behav. 2012;6:568–83.
Jagust W, Gitcho A, Sun F, Kuczynski B, Mungas D, Haan M. Brain imaging evidence of preclinical Alzheimer's disease in normal aging. Ann Neurol. 2006;59:673–81.
Landau SM, Harvey D, Madison CM, Koeppe RA, Reiman EM, Foster NL, et al. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32:1207–18.
Shokouhi S, Claassen D, Kang H, Ding Z, Rogers B, Mishra A, et al. Longitudinal progression of cognitive decline correlates with changes in the spatial pattern of brain 18F-FDG PET. J Nucl Med. 2013;54:1564–9.
Alexopoulos P, Sorg C, Forschler A, Grimmer T, Skokou M, Wohlschlager A, et al. Perfusion abnormalities in mild cognitive impairment and mild dementia in Alzheimer’s disease measured by pulsed arterial spin labeling MRI. Eur Arch Psychiatry Clin Neurosci. 2012;262:69–77.
Jueptner M, Weiller C. Review: does measurement of regional cerebral blood flow reflect synaptic activity? Implications for PET and fMRI. Neuroimage. 1995;2:148–56.
Bozzao A, Floris R, Baviera ME, Apruzzese A, Simonetti G. Diffusion and perfusion MR imaging in cases of Alzheimer's disease: correlations with cortical atrophy and lesion load. AJNR Am J Neuroradiol. 2001;22:1030–6.
Shimizu S, Zhang Y, Laxamana J, Miller BL, Kramer JH, Weiner MW, et al. Concordance and discordance between brain perfusion and atrophy in frontotemporal dementia. Brain Imaging Behav. 2010;4:46–54.
Hulette CM, Welsh-Bohmer KA, Murray MG, Saunders AM, Mash DC, McIntyre LM. Neuropathological and neuropsychological changes in “normal” aging: evidence for preclinical Alzheimer disease in cognitively normal individuals. J Neuropathol Exp Neurol. 1998;57:1168–74.
Pearl GS. Diagnosis of Alzheimer's disease in a community hospital-based brain bank program. South Med J. 1997;90:720–2.
Petersen RC, Parisi JE, Dickson DW, Johnson KA, Knopman DS, Boeve BF, et al. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol. 2006;63:665–72.
Acknowledgments
We thank Avid Radiopharmaceuticals Inc. (Philadelphia, PA, USA) for providing the precursor for the preparation of 18F-florbetapir.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was carried out with financial support from the National Research Program for Biopharmaceuticals, National Science Council, Taiwan (MOST 103-2314-B-182A-009, 104-2314-B-182A-083-MY2, 103-2325-B-182A-009) and grants from the Research Fund of Chang Gung Memorial Hospital (CMRPG390793).
Conflicts of Interest
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study. In addition, the next of kin or guardians of AD and MCI patients also gave their written informed consent if the patients could not comprehend the study protocol or they could not sign their name clearly.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
(DOCX 26 kb)
Supplementary Table 2
(DOCX 43 kb)
Supplementary Table 3
(DOCX 63 kb)
Supplementary Table 4
(DOCX 32 kb)
Supplementary Table 5
(DOCX 23 kb)
Rights and permissions
About this article
Cite this article
Lin, KJ., Hsiao, IT., Hsu, JL. et al. Imaging characteristic of dual-phase 18F-florbetapir (AV-45/Amyvid) PET for the concomitant detection of perfusion deficits and beta-amyloid deposition in Alzheimer’s disease and mild cognitive impairment. Eur J Nucl Med Mol Imaging 43, 1304–1314 (2016). https://doi.org/10.1007/s00259-016-3359-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-016-3359-8