Abstract
Purpose
Typical and atypical carcinoids (TC and AC) represent 20 – 25 % of all neuroendocrine tumours. No standard therapeutic approach is available for patients with advanced disease. The aim of this phase II study was to investigate the efficacy and safety of peptide receptor radionuclide therapy with 177Lu-DOTATATE (Lu-PRRT) and the role of thyroid transcription factor 1 (TTF-1) and 18F-FDG PET as prognostic factors in patients with advanced TC or AC.
Methods
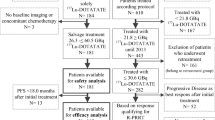
A total of 34 consecutive patients with radiologically documented progressive disease were treated with Lu-PRRT at a therapeutic cumulative activity of 18.5 or 27.8 GBq in four or five cycles according to the patient’s kidney function and bone marrow reserve. Information on TTF-1 was available in all patients. FDG PET studies prior to Lu-PRRT were available in 29 patients.
Results
The median follow-up was 29 months (range 7 – 69 months). The disease control rate (DCR) in patients with TC was 80 %: 6 % complete response, 27 % partial response and 47 % stable disease. The median progression-free survival (mPFS) was 20.1 months (95 % CI 11.8 – 26.8 months). Stable disease was achieved in 47 % of patients with AC with a mPFS of 15.7 months (95 % CI 10.6 – 25.9 months). No major acute or delayed toxicity occurred in either group or with either cumulative activity. mPFS in patients with TTF-1-negative TC was 26.3 months (95 % CI 12.9 – 45.2 months), but in patients with TTF-1-positive TC mPFS was 7.2 months (4.2 – 14.0 months; p = 0.0009). FDG PET was negative in 13 patients (10 TC and 3 AC) and positive in 16 patients (4 TC and 12 AC). The mPFS in the FDG PET-negative group was 26.4 months (95 % CI 14.2 – 48.9 months) and 15.3 months (11.7 – 31.1 months) in the FDG PET-positive group.
Conclusion
Lu-PRRT showed antitumour activity in terms of DCR and PFS and proved safe, even in patients with a higher risk of side effects. TTF-1 would appear to be a prognostic factor. FDG PET positivity in bronchial carcinoids is a hallmark of aggressive tumour and is more frequent in patients with AC than in those with TC.


Similar content being viewed by others
References
Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC (editors) World HealthOrganization Classification of Tumours. Pathology and genetics of tumours of thelung, pleura, thymus and feart. Lyon: IARC Press; 2004. http://www.iarc.fr/en/publications/pdfs-online/pat-gen/bb10/BB10.pdf.
Caplin ME, Baudin E, Ferolla P, Filosso P, Garcia-Yuste M, Lim E, et al. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann Oncol. 2015;26:1604–20.
Sturm N, Lantuéjoul S, Laverrière MH, Papotti M, Brichon PY, Brambilla C, et al. Thyroid transcription factor 1 and cytokeratins 1, 5, 10, 14 (34betaE12) expression in basaloid and large-cell neuroendocrine carcinomas of the lung. Hum Pathol. 2001;32:918–25.
Folpe AL, Gown AM, Lamps LW, Garcia R, Dail DH, Zarbo RJ, et al. Thyroid transcription factor-1: immunohistochemical evaluation in pulmonary neuroendocrine tumors. Mod Pathol. 1999;12:5–8.
Righi L, Volante M, Tavaglione V, Billè A, Daniele L, Angusti T, et al. Somatostatin receptor tissue distribution in lung neuroendocrine tumours: a clinicopathologic and immunohistochemical study of 218 ‘clinically aggressive’ cases. Ann Oncol. 2010;21:548–55.
Granberg D, Sundin A, Janson ET, Oberg K, Skogseid B, Westlin JE. Octreoscan in patients with bronchial carcinoid tumors. Clin Endocrinol. 2003;59:793–9.
Gabriel M, Decristoforo C, Kendler D, Dobrozemsky G, Heute D, Uprimny C, et al. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med. 2007;48:508–18.
Rivera MP, Detterbeck F, Mehta AC. Diagnosis of lung cancer: the guidelines. Chest. 2003;123(1 Suppl):129S–36.
Lim E, Goldstraw P, Nicholson AG, Travis WD, Jett JR, Ferolla P, et al. Proceedings of the IASLC International Workshop on Advances in Pulmonary Neuroendocrine Tumors 2007. J Thorac Oncol. 2008;3:1194–201.
Pavel ME, Hainsworth JD, Baudin E, Peeters M, Hörsch D, Winkler RE, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378:2005–12.
Kwekkeboom DJ, Mueller-Brand J, Paganelli G, Anthony LB, Pauwels S, Kvols LK, et al. Overview of results of peptide receptor radionuclide therapy with 3 radiolabeled somatostatin analogs. J Nucl Med. 2005;46 Suppl 1:62S–6.
Bodei L, Cremonesi M, Grana CM, Fazio N, Iodice S, Baio SM, et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE: the IEO phase I-II study. Eur J Nucl Med Mol Imaging. 2011;38:2125–35.
Bodei L, Cremonesi M, Ferrari M, Pacifici M, Grana CM, Bartolomei M, et al. Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90Y-DOTATOC and 177Lu-DOTATATE: the role of associated risk factors. Eur J Nucl Med Mol Imaging. 2008;35:1847–56.
Ezziddin S, Khalaf F, Vanezi M, Haslerud T, Mayer K, Al Zreiqat A, et al. Outcome of peptide receptor radionuclide therapy with 177Lu-octreotate in advanced grade 1/2 pancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2014;41:925–33.
Sabet A, Dautzenberg K, Haslerud T, Aouf A, Sabet A, Simon B, et al. Specific efficacy of peptide receptor radionuclide therapy with (177)Lu-octreotate in advanced neuroendocrine tumours of the small intestine. Eur J Nucl Med Mol Imaging. 2015;42:1238–46.
Sabet A, Ezziddin K, Pape UF, Reichman K, Haslerud T, Ahmadzadehfar H, et al. Accurate assessment of long-term nephrotoxicity after peptide receptor radionuclide therapy with (177)Lu-octreotate. Eur J Nucl Med Mol Imaging. 2014;41:505–10.
van Essen M, Krenning EP, Bakker WH, de Herder WW, van Aken MO, Kwekkeboom DJ. Peptide receptor radionuclide therapy with 177Lu-octreotate in patients with foregut carcinoid tumors of bronchial, gastric and thymic origin. Eur J Nucl Med Mol Imaging. 2007;34:1219–27.
Bodei L, Cremonesi M, Kidd M, Grana CM, Severi S, Modlin IM, et al. Peptide receptor radionuclide therapy for advanced neuroendocrine tumors. Thorac Surg Clin. 2014;24:333–49.
Binderup T, Knigge U, Loft A, Federspiel B, Kjaer A. 18F-fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin Cancer Res. 2010;16:978–85.
Severi S, Nanni O, Bodei L, Sansovini M, Ianniello A, Nicoletti S, et al. Role of 18FDG PET/CT in patients treated with 177Lu-DOTATATE for advanced differentiated neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2013;40:881–8.
Sandström M, Garske-Román U, Granberg D, Johansson S, Widström C, Eriksson B, et al. Individualized dosimetry of kidney and bone marrow in patients undergoing 177Lu-DOTA-octreotate treatment. J Nucl Med. 2013;54(1):33–41.
Paganelli G, Sansovini M, Ambrosetti A, Severi S, Monti M, Scarpi E, et al. 177Lu-Dota-octreotate radionuclide therapy of advanced gastrointestinal neuroendocrine tumors: results from a phase II study. Eur J Nucl Med Mol Imaging. 2014;41(10):1845–51.
Bergsma H, Konijnenberg MW, Kam BL, Teunissen JJ, Kooij PP, de Herder WW, et al. Subacute haematotoxicity after PRRT with 177Lu-DOTA-octreotate: prognostic factors, incidence and course. Eur J Nucl Med Mol Imaging. 2015. doi:10.1007/s00259-015-3193-4
Breeman WA, de Blois E, Bakker WH. Radiolabeling DOTA peptides with 90Y and 177Lu to a high specific activity. In: Chinol M, Paganelli G, editors. Radionuclide peptide cancer therapy. New York: Taylor & Francis; 2006. p. 119–26.
Green S, Weiss GR. Southwest Oncology Group standard response criteria, endpoint definitions and toxicity criteria. Invest New Drugs. 1992;10:239–53.
van Vliet EI, Krenning EP, Teunissen JJ, Bergsma H, Kam BL, Kwekkeboom DJ. Comparison of response evaluation in patients with gastroenteropancreatic and thoracic neuroendocrine tumors after treatment with [177Lu-DOTA0, Tyr3] octreotate. J Nucl Med. 2013;54:1689–96.
Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, Version 3.0, DCTD, NCI, NIH, DHHS31 March 2003. 2006. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
Kaplan EL, Meier P. Nonparametric estimation for incomplete observation. J Am Stat Assoc. 1958;53:457–81.
Lawless JS. Statistical models and methods for life-time data. New York: Wiley; 1982.
Granberg D, Eriksson B, Wilander E, Grimfjärd P, Fjällskog ML, Oberg K, et al. Experience in treatment of metastatic pulmonary carcinoid tumors. Ann Oncol. 2001;12:1383–91.
Claringbold PG, Brayshaw PA, Price RA, Turner JH. Phase II study of radiopeptide 177Lu-octreotate and capecitabine therapy of progressive disseminated neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2011;38:302–11.
Kashyap R, Hofman MS, Michael M, Kong G, Akhurst T, Eu P, et al. Favourable outcomes of 177Lu-octreotate peptide receptor chemoradionuclide therapy in patients with FDG-avid neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2015;42(2):176–85.
Acknowledgments
The authors thank Ursula Elbling for editing the manuscript. This study was partially supported by AIRC – Associazione Italiana per la Ricerca sul Cancro (grant number: IG10679).
Authors’ contributions
S.S. coordinated the various stages of the project and was involved in the preparation, treatment and follow-up of patients. A.I., M.S., S.N., C.M.G., K.M. were involved in the enrolment, preparation, treatment and follow-up of patients. P.C. performed the diagnostic and therapeutic scintigraphy scans. A.B. and L.A. were involved in the preparation, treatment and follow-up of patients. V.D.I. prepared the radiopharmaceutical. A.S. was responsible for imaging quality control. M.M. was responsible for data collection. E.S. performed the statistical analysis. A.I. and G.P. drafted the manuscript. G.P. reviewed the manuscript for important intellectual content. All authors read and approved the final version of the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None.
Conflict of interest
None.
Ethical approval
The protocol was approved by the Ethics Committee of the Wide Catchment Area of Romagna-IRST (CEIIAV) and by the competent Italian regulatory authorities. The study was conducted in accordance with the principles of the Declaration of Helsinki and good clinical practice guidelines.
Informed consent
All patients gave written informed consent.
Rights and permissions
About this article
Cite this article
Ianniello, A., Sansovini, M., Severi, S. et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE in advanced bronchial carcinoids: prognostic role of thyroid transcription factor 1 and 18F-FDG PET. Eur J Nucl Med Mol Imaging 43, 1040–1046 (2016). https://doi.org/10.1007/s00259-015-3262-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-015-3262-8