Abstract
Purpose
The frontal lobes play a crucial role in micturition control. However, no reports exist on the functional role of distinct frontal brain regions in urinary incontinence (UIC) in patients with a neurodegenerative damage of the frontal lobe. The aim of the present study was therefore to explore if functional brain lesions were associated with UIC in patients suffering from frontotemporal lobar degenerations (FTLD).
Methods
Forty-four patients, including eight incontinent subjects, underwent cranial positron emission tomography scanning with 18F-fluoro-2-deoxy-glucose (18F-FDG PET) to assess the relative metabolic rate of glucose (rCMRglc). Group comparisons of rCMRglc were conducted in SPM2 to identify brain regions where the group of incontinent patients (FTLD+UIC) had significant alterations compared with the group without UIC (FTLD−UIC).
Results
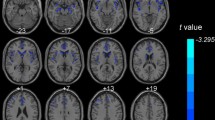
At the stringent statistical threshold of p < 0.05, corrected for multiple comparisons according to the family-wise error rate, the statistical analysis revealed two significant right-hemispheric hypometabolic clusters located in the premotor/anterior cingulate cortex and the putamen/claustrum/insula. No hypermetabolic regions were found.
Conclusions
The present study is the first to provide evidence for brain functional alterations involved in the occurrence of UIC in FTLD. These results provide an important piece of evidence to the understanding of a particularly distressing autonomic nervous system symptom of dementia.

Similar content being viewed by others
References
Andrew J, Nathan PW. Lesions on the anterior frontal lobes and disturbances of micturition and defaecation. Brain 1964;87:233–62.
Athwal BS, Berkley KJ, Hussain I, et al. Brain responses to changes in bladder volume and urge to void in healthy men. Brain 2001;124:369–77.
Bennett DA, Gilley DW, Lee S, et al. White matter changes: neurobehavioral manifestations of Binswanger’s disease and clinical correlates in Alzheimer’s disease. Dementia 1994;5:148–52.
Blok BF, Willemsen AT, Holstege G. A PET study on brain control of micturition in humans. Brain 1997;120(Pt 1):111–21.
Boecker H, Ceballos-Baumann AO, Volk D, et al. Metabolic alterations in patients with Parkinson disease and visual hallucinations. Arch Neurol 2007;64:984–8.
Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 2000;4:215–22.
Critchley HD, Wiens S, Rotshtein P, et al. Neural systems supporting interoceptive awareness. Nat Neurosci 2004;7:189–95.
Diehl-Schmid J, Grimmer T, Drzezga A, et al. Longitudinal changes of cerebral glucose metabolism in semantic dementia. Dement Geriatr Cogn Disord 2006;22:346–51.
Diehl-Schmid J, Grimmer T, Drzezga A, et al. Decline of cerebral glucose metabolism in frontotemporal dementia: a longitudinal 18F-FDG-PET-study. Neurobiol Aging 2007;28:42–50.
Drzezga A, Lautenschlager N, Siebner H, et al. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer’s disease: a PET follow-up study. Eur J Nucl Med Mol Imaging 2003;30:1104–13.
Drzezga A, Riemenschneider M, Strassner B, et al. Cerebral glucose metabolism in patients with AD and different APOE genotypes. Neurology 2005;64:102–7.
Dubois B, Slachevsky A, Litvan I, et al. The FAB: a Frontal Assessment Battery at bedside. Neurology 2000;55:1621–6.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98.
Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol 2004;55:335–46.
Griffiths D. Clinical studies of cerebral and urinary tract function in elderly people with urinary incontinence. Behav Brain Res 1998;92:151–5.
Griffiths D, Tadic SD, Schaefer W, et al. Cerebral control of the bladder in normal and urge-incontinent women. Neuroimage 2007;37:1–7.
Harvey RJ, Skelton-Robinson M, Rossor MN. The prevalence and causes of dementia in people under the age of 65 years. J Neurol Neurosurg Psychiatry 2003;74:1206–9.
Herholz K, Salmon E, Perani D, et al. Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. Neuroimage 2002;17:302–16.
Hirono N, Kitagaki H, Kazui H, et al. Impact of white matter changes on clinical manifestation of Alzheimer's disease: a quantitative study. Stroke 2000;31:2182–8.
Holstege G, Griffiths D, de Wall H, et al. Anatomical and physiological observations on supraspinal control of bladder and urethral sphincter muscles in the cat. J Comp Neurol 1986;250:449–61.
Hughes CP, Berg L, Danziger WL, et al. A new clinical scale for the staging of dementia. Br J Psychiatry 1982;140:566–72.
Kavia RB, Dasgupta R, Fowler CJ. Functional imaging and the central control of the bladder. J Comp Neurol 2005;493:27–32.
Kertesz A, Davidson W, Fox H. Frontal behavioral inventory: diagnostic criteria for frontal lobe dementia. Can J Neurol Sci 1997;24:29–36.
Kitta T, Kakizaki H, Furuno T, et al. Brain activation during detrusor overactivity in patients with Parkinson’s disease: a positron emission tomography study. J Urol 2006;175:994–8.
Kono AK, Ishii K, Sofue K, et al. Fully automatic differential diagnosis system for dementia with Lewy bodies and Alzheimer’s disease using FDG-PET and 3D-SSP. Eur J Nucl Med Mol Imaging 2007;34:1490–7.
Minoshima S, Foster NL, Sima AA, et al. Alzheimer’s disease versus dementia with Lewy bodies: cerebral metabolic distinction with autopsy confirmation. Ann Neurol 2001;50:358–65.
Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998;51:1546–54.
Nour S, Svarer C, Kristensen JK, et al. Cerebral activation during micturition in normal men. Brain 2000;123(Pt 4):781–9.
Perneczky R, Diehl-Schmid J, Pohl C, et al. Non-fluent progressive aphasia: Cerebral metabolic patterns and brain reserve. Brain Res 2007;1133:178–85.
Perneczky R, Wagenpfeil S, Komossa K, et al. Mapping scores onto stages: mini-mental state examination and clinical dementia rating. Am J Geriatr Psychiatry 2006;14:139–44.
Pool JL, Ransohoff J. Autonomic effects on stimulating rostral portion of cingulate gyri in man. J Neurophysiol 1949;12:385–92.
Ratnavalli E, Brayne C, Dawson K, et al. The prevalence of frontotemporal dementia. Neurology 2002;58:1615–21.
Reitan R. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills 1985;8:271–6.
Sakakibara R, Uchida Y, Uchiyama T, et al. Reduced cerebellar vermis activation during urinary storage and micturition in multiple system atrophy: 99mTc-labelled ECD SPECT study. Eur J Neurol 2004;11:705–8.
Talairach J, Tournoux P. Co-planar stereotactical atlas of the human brain: 3-dimensional proportional system - an approach to cerebral imaging. Thieme Medical Publishers, New York, 1988.
Thalmann B, Monsch A, CERAD. The Consortium to Establish a Registry for Alzheimer’s Disease. Neuropsychologische Testbatterie. Memory Clinic Basel, Basel, 1997.
Walters K, Iliffe S, Tai SS, et al. Assessing needs from patient, carer and professional perspectives: the Camberwell Assessment of Need for Elderly people in primary care. Age Ageing 2000;29:505–10.
Acknowledgements
Dr. Diehl-Schmid received KKF and HWP-II grants from the Technical University Munich. However, the sponsor did neither play a role in design and conduct of the study, collection, management, analysis and interpretation of the data nor in the preparation, review or approval of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Robert Perneczky and Janine Diehl-Schmid contributed equally.
Rights and permissions
About this article
Cite this article
Perneczky, R., Diehl-Schmid, J., Förstl, H. et al. Urinary incontinence and its functional anatomy in frontotemporal lobar degenerations. Eur J Nucl Med Mol Imaging 35, 605–610 (2008). https://doi.org/10.1007/s00259-007-0626-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-007-0626-8